August
1st
- made electrocompetent E.coli rosetta cells.
- prepared cultures of K1319042 and K131026
2nd
- tested the OD measurement device and compared it to the spectrophotometer and the plate reader.
- tested K131026 and K1319042 for fluorescence in the plate reader
- did a heat shock transformation of I746909 into NEB TOP 10 cells
- did an electroshock transformation of pET17-Gal3 into E.coli rosetta
3rd
- OD measurements of the iGEM device in comparison to the spectrophotometer were taken.
- cryo cultures of K131026 and K1319042 were prepared
- master plates of Gal3 #1-#10 and I746909 #1-#4 and overnight cultures
4th
- made cryo stocks of K1319042 and K131026 in NEB/BL21/DH5alpha, I746909 in BL21 and pET17-His-SNAP-YFP-Gal3 in E. coli rosetta (DE3), respectively.
- made plasmid prep, most of them using 1.5 mL culture medium, and eluted with 1x 50 µL of ddH2O. The resulting DNA concentrations are shown below.
| combination | concentration [ng/µl]
|
| I746909 BL21 #1 | 73.5
|
| I746909 BL21 #2 | 45
|
| I746909 BL21 #3 | 49
|
| K1319042 DH5alpha | 60
|
| K131026 DH5alpha | 150
|
| pET17-Gal3 #1 | 30.5
|
| pET17-Gal3 #2 | 6.4
|
| pET17-Gal3 #3 | 6.3
|
| pET17-Gal3 #4 | 9.4
|
| pET17-Gal3 #5 | 10.1
|
| pET17-Gal3 #6 | 8.2
|
| pET17-Gal3 #7 | 13.8
|
| pET17-Gal3 #8 | 6.9
|
| pET17-Gal3 #9 | 10.2
|
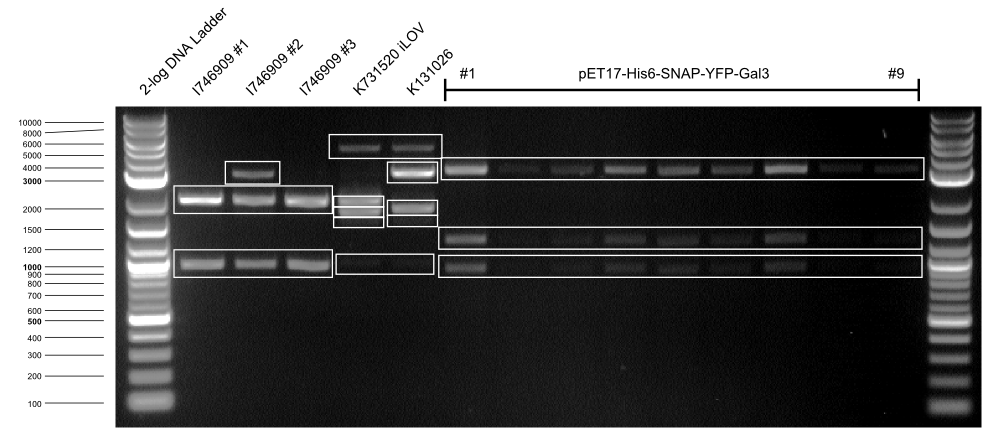
To confirm the quality of pET17-Gal3 transformations, the purified plasmids were tested by carrying out a digest. Results are shown in the below picture and table.
| combination | cut products[bp]
|
| I746909 BL21 #1 | 2029, 947
|
| K1319042 DH5alpha | 2029, 1780
|
| K131026 DH5alpha | 2029, 1848
|
| pET17-Gal3 #1 | 3086, 923, 1262
|
All pET17-Gal3 clones were positive and clone #1 was selected for further experiments.
- prepared an over night cultur of K1319042 for chips
5th
- assembly of a VR=2.5 L bioreactor for cultivation of a 1 L expression culture.
- Two precultures of 20 mL LB+A were inoculated at 19:00
- transformation of K746909 into BL21 cells and K1319000 into NEB10β cells.
- made Chips with K1319042 in HM. Images were taken every 30 min with the Geldoc
- made alliquots of HM, 1 L HM + glucose + supplements and 500 ml LB
6th
- transformation of J04450 in pSB1K3 and pSB1A3 in NEB10β cells.
- They also did a plasmid prep of J04450 in pSB1C3 and Flo's vectors.
- made precultures of NEB10βand DH5 alpha cells
- inoculation of the fermenter at 11:40, and induced the fermentation of pET17-Gal3. The fermentation is expected to run 24 h.
7th
- made media for Pseudomonas flourescens
- Nutrient Broth
- Pseudomonas-F
- Pseudomonas-P
- made 2 L LB
8th
- prepared 2x 60 ml (LB + cam + IPTG) with K1319042
- prepared 5 ml K131026 and C0179
- made SDS page
9th
- made a plate reader experiment with K131026 in LB and LB + HM
11th
- plated on LB + antibiotics
- K131026
- I746909
- K13190042
- I04450 in pSB1C3
- I04450 in pSB1A3
- I04450 in pSB1K3
- pSEVA construct (pSEVA 641_FP pSEVA 234-LasR)
13th
- made chips with
- K131026 and I746909 in LB and HM
- K1319042 and the pSEVA two plasmid construct in HM
- Images were taken every 30 min with the Geldoc
18th
- made over night cultures of I746909 and K131026 in LB, TB, 2x HM+
- plated 3x J23101.E0240
19th
- made Chips with K131026 and I746909 in HM. Images were taken every 30 min with the Geldoc
- made PCR of J23101.E0240, K1319000, K1319001, K1319002 and run a 1,2 % agarose gel
20th
- repeat PCR for REACh1 and J23101.E0240 and run a gel
- plasmid prep of pSEVA BfsB, pSEVA lasR and I746909
24th
- made 2.5 L LB
- made chips of K131026 in NEB , DH5α and BL21 in LB and additionally K131026 in DH5α in HM+. Images were taken every 30 min with our own device
25th
- did a plasmid restriction of I20260 (EcoRI,PstI), J23115 (EcoRI, SpeI), K516032 (XbaI,PstI), and J23101 (EcoRI, SpeI)
- tested the growth of Pseudomonas fluorescens in different liquid media for high OD and strong fluorescence. She tested Standard I medium, Cetrimide medium and Pseudomonas-F medium, and Pseudomonas-F medium supplemented with 300 µL Fe3+ in 500 mL flasks with a filling volume of 30 mL. The flasks were inoculated with P. fluorescens cells on Standard I agar, and incubated at 30 °C at 250 rpm.
- prepared over night cultures of K131026 in DH5α and NEB for chips
- prepared 2x 5 ml of pSB1C3, psB3K3 and pSB1A2 for plasmid prep
26th
- ligation of J23115 and K516032 to J23115.K516032, and J23101 and K516032 to J23101.K516032, respectively.
- plasmid prep of I20260, K516032 and B0034
- restriction of plasmids I20260, K516032, B0034 with EcoRI and PstI
- gel with restricted the I20260, K516032 and B0034 was run
- purification of vector backbones pSB1A2, pSB3K3 and pSB1C3
- restriciton of synthesized TEV protease with EcoRI and PstI
- qualitatively tested the Pseudomonas fluorescens that had grown over night for OD and fluorescence. She determined that Pseudomonas-F medium is the most adequate for the cultivation of the strain we use, since both OD and fluorescence were best in the flask containing the respective medium. Growth in the Pseudomonas-F medium supplemented with 300 µg/L Fe3+ was weaker, however, fluorescence was also successfully suppressed.
- made chips with K131026 in DH5α and NEB, in LB and LB + 10 % glycerol. Images were taken with our own device every 30 min.
- plasmid prep of the back bones, restriction and gel purification
27th
- transformation of some BioBricks
- ligation of J23101.K516032 into pSB3K3 and J23115.K516032 into pSB3K3 and K1319004 into pSB1C3
- transformation of K1319004 into pUC and pSB1C3, and J04450 into pSB1K3 and pSB1A3, respectively
28th
- transformation of some BioBricks
back to Wetlab main page
|
 "
"