Team:SUSTC-Shenzhen/Notebook/CRISPR/Verifications-for-struggles-for-our-second-time-Fill-in
From 2014.igem.org
| Line 11: | Line 11: | ||
{{:Team:SUSTC-Shenzhen/templates/notebook-main| | {{:Team:SUSTC-Shenzhen/templates/notebook-main| | ||
name=Verifications for 'struggles for our second- time Fill-in'| | name=Verifications for 'struggles for our second- time Fill-in'| | ||
| - | date=2014/8/ | + | date=2014/8/14~2014/8/16| |
goal=}} | goal=}} | ||
| - | == | + | ==Materials== |
| + | |||
| + | Enzymes: EcoRI & MfeI Buffer: Buffer 2.1 | ||
| + | |||
| + | ==Methods== | ||
| + | |||
| + | === Digestion system:(ul) === | ||
| + | |||
| + | {|class="table" | ||
| + | !MfeI & EcoRI | ||
| + | !Total volume | ||
| + | !DNA | ||
| + | !Buffer 2.1 | ||
| + | !MfeI | ||
| + | !EcoRI | ||
| + | !ddH2O | ||
| + | |- | ||
| + | |G (1) 689ng/ul | ||
| + | |10 | ||
| + | |2.2 | ||
| + | |1 | ||
| + | |1.5 | ||
| + | |1.5 | ||
| + | |3.8 | ||
| + | |- | ||
| + | |G (2) 754ng/ul | ||
| + | |10 | ||
| + | |1.9 | ||
| + | |1 | ||
| + | |1.5 | ||
| + | |1.5 | ||
| + | |4.1 | ||
| + | |} | ||
| + | |||
| + | {|class="table" | ||
| + | !MfeI / EcoRI | ||
| + | !Total volume | ||
| + | !DNA | ||
| + | !Buffer 2.1 | ||
| + | !MfeI / EcoRI | ||
| + | !ddH2O | ||
| + | |- | ||
| + | |G (1) 689ng/ul | ||
| + | |10 | ||
| + | |2.2 | ||
| + | |1 | ||
| + | |1.5 | ||
| + | |5.3 | ||
| + | |- | ||
| + | |G (2) 754ng/ul | ||
| + | |10 | ||
| + | |1.9 | ||
| + | |1 | ||
| + | |1.5 | ||
| + | |5.6 | ||
| + | |} | ||
| + | |||
| + | Incubate at 37°C for 4hours | ||
| + | |||
| + | === Results of electrophoresis=== | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/7/7c/SUSTC-Shenzhen-Verifications-for-succeed-Fill-in-plasmid.jpg}} | ||
| + | |||
| + | The bands of G1 were to compliated to analyze. | ||
| + | For G2, according to our assumption, If the EcoRI site had been filled out, the profile of its bands should be congruent to that of the original plasmid; if not, it should be aligned to that of the M digestion. From the picture, we can see that except for the abnormal bands of double digestion (EcoRI and MfeI), the other DNA bands along single digestion tracks could roughly imply that the EcoRI site had been removed from the plasmid by Fill-in. Because the bands of EcoRI digestion were similar to those of the original plasmid which hadn’t been digested by any restriction enzymes. While the very band of MfeI digestion was obviously behind those of E digestion and original plasmid, indicating that digestion with MfeI had linearized the plasmid. | ||
| + | |||
| + | |||
| + | |||
| + | |||
After analyzing, we extrapolated that G 5,6 might be a successful one, since this plasmid only digested with EcoRI hadn’t been cut into two fragments and seemed similar to the original plasmid. Other situations (double digestion, digestion with MfeI ) were too complicated to infer any valid information. | After analyzing, we extrapolated that G 5,6 might be a successful one, since this plasmid only digested with EcoRI hadn’t been cut into two fragments and seemed similar to the original plasmid. Other situations (double digestion, digestion with MfeI ) were too complicated to infer any valid information. | ||
Revision as of 03:59, 17 October 2014
Notebook
Elements of the endeavor.
Verifications for 'struggles for our second- time Fill-in'
2014/8/14~2014/8/16
Materials
Enzymes: EcoRI & MfeI Buffer: Buffer 2.1
Methods
Digestion system:(ul)
| MfeI & EcoRI | Total volume | DNA | Buffer 2.1 | MfeI | EcoRI | ddH2O |
|---|---|---|---|---|---|---|
| G (1) 689ng/ul | 10 | 2.2 | 1 | 1.5 | 1.5 | 3.8 |
| G (2) 754ng/ul | 10 | 1.9 | 1 | 1.5 | 1.5 | 4.1 |
| MfeI / EcoRI | Total volume | DNA | Buffer 2.1 | MfeI / EcoRI | ddH2O |
|---|---|---|---|---|---|
| G (1) 689ng/ul | 10 | 2.2 | 1 | 1.5 | 5.3 |
| G (2) 754ng/ul | 10 | 1.9 | 1 | 1.5 | 5.6 |
Incubate at 37°C for 4hours
Results of electrophoresis
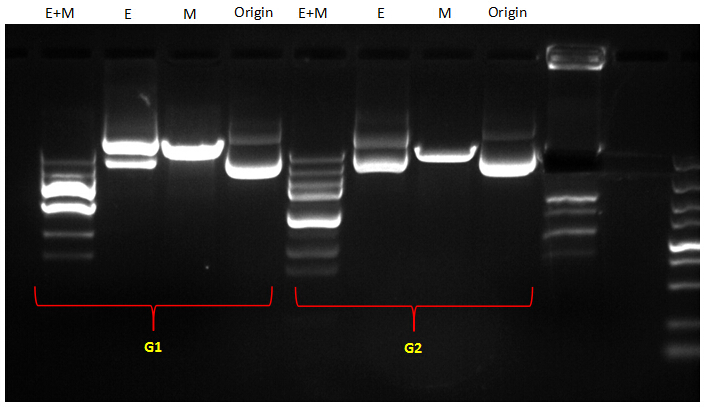
The bands of G1 were to compliated to analyze. For G2, according to our assumption, If the EcoRI site had been filled out, the profile of its bands should be congruent to that of the original plasmid; if not, it should be aligned to that of the M digestion. From the picture, we can see that except for the abnormal bands of double digestion (EcoRI and MfeI), the other DNA bands along single digestion tracks could roughly imply that the EcoRI site had been removed from the plasmid by Fill-in. Because the bands of EcoRI digestion were similar to those of the original plasmid which hadn’t been digested by any restriction enzymes. While the very band of MfeI digestion was obviously behind those of E digestion and original plasmid, indicating that digestion with MfeI had linearized the plasmid.
After analyzing, we extrapolated that G 5,6 might be a successful one, since this plasmid only digested with EcoRI hadn’t been cut into two fragments and seemed similar to the original plasmid. Other situations (double digestion, digestion with MfeI ) were too complicated to infer any valid information.
 "
"