Team:Paris Saclay/Notebook/September/3
From 2014.igem.org
(→LabWork) |
m (→Wednesday 3rd September) |
||
| Line 17: | Line 17: | ||
Electrophoresis of the PCR made [https://2014.igem.org/Team:Paris_Saclay/Notebook/September/2#Limonene_synthase_PCR Yesterday] | Electrophoresis of the PCR made [https://2014.igem.org/Team:Paris_Saclay/Notebook/September/2#Limonene_synthase_PCR Yesterday] | ||
| - | [[File:0309 PCR LS + extraction pPS5.jpg|400px]] | + | [[File:0309 PCR LS + extraction pPS5.jpg|400px|center]] |
well 2-3 = PCR with Dream taq or Vent taq | well 2-3 = PCR with Dream taq or Vent taq | ||
| Line 28: | Line 28: | ||
Electrophoresis after purification | Electrophoresis after purification | ||
| - | [[File:0309 purif LS.jpg|400px]] | + | [[File:0309 purif LS.jpg|400px|center]] |
| Line 105: | Line 105: | ||
Electrophoresis | Electrophoresis | ||
| - | [[File:0309 digestion SalI pPS5.jpg|500px]] | + | [[File:0309 digestion SalI pPS5.jpg|500px|center]] |
We saw that we have something strang with our plasmid but we wil check it tomorrow with a good ladder (the ladder here was normally on the well 1) | We saw that we have something strang with our plasmid but we wil check it tomorrow with a good ladder (the ladder here was normally on the well 1) | ||
| Line 131: | Line 131: | ||
|} | |} | ||
| - | [[File:0309 digestion HindIII pPS3 4.jpg|500px]] | + | [[File:0309 digestion HindIII pPS3 4.jpg|500px|center]] |
well 1 = ladder | well 1 = ladder | ||
Latest revision as of 12:50, 14 October 2014
Contents |
Wednesday 3rd September
LabWork
Construction of the fusion protein
We made a classic exttraction of plasmids from the liquid cultures.
Check by electrophoresis if it worked and send it for sequencing.
Lemon scent
by Mélanie
PCR LS
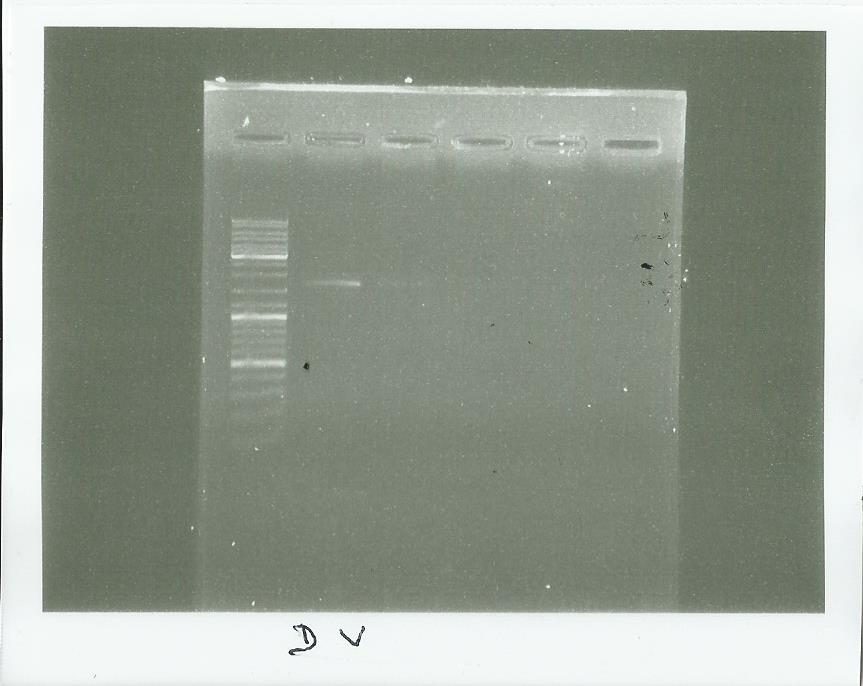
Electrophoresis of the PCR made Yesterday
well 2-3 = PCR with Dream taq or Vent taq
(well 4-5 = checking of the pps5 Extraction)
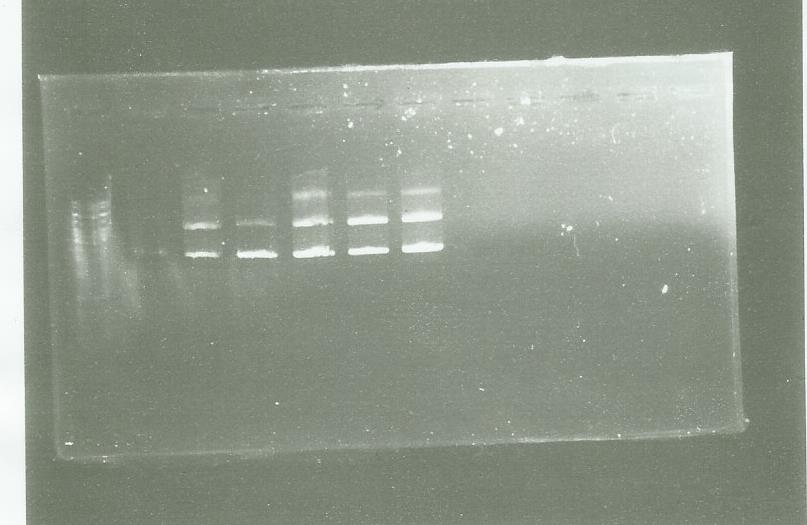
The PCR have success so I use the PCR clean up kit to purify it
Electrophoresis after purification
Digestion
In order to clone LS in pPSI, i Digest it by PacI
| component | volume |
|---|---|
| H2O | 3μl |
| buffer | 1μl |
| PacI | 1μl |
| PCR purify | 5μl |
Ligation
We already have some pPSI digested and dephosphorelated
So I do a ligation:
| component | volume |
|---|---|
| H2O | 5μl |
| buffer | 2μl |
| ligase | 1μl |
| pPSI | 2μl |
| LS PCR | 10μl |
2 hours at room temperature and over night at 4°
pPS5
Plasmid exctraction using the kit (picture is here)
digestion by SalI
| component | volume |
|---|---|
| H2O | 11μl |
| buffer | 5μl |
| SalI | 2μl |
| pPS5 | 30μl |
Electrophoresis
We saw that we have something strang with our plasmid but we wil check it tomorrow with a good ladder (the ladder here was normally on the well 1)
pPS3 and pPS4
We digest the plasmid by HindIII to direct our insert
| component | volume |
|---|---|
| H2O | 6μl |
| buffer | 1μl |
| HindIII | 1μl |
| pPS3/4 | 2μl |
well 1 = ladder well 2-3-4 = pPS3 Well 5-6-7 = pPS4
results are very strange
Photo of the Day
[http://iramis.cea.fr/llb/Phocea/Pisp/visu.php?id=35&uid=%20alexei.grinbaum Alexei Grinbaum] , one of those few we have interviewed during this month of september.
To learn more about the ethic aspect of our project, just click here
 "
"