Team:ETH Zurich/lab/chip
From 2014.igem.org
(→Mold Design and 3D Print Exchange) |
(→Mold Design and 3D Print Exchange) |
||
| Line 44: | Line 44: | ||
'''All mesh files designed during the project will be made available at the [http://3dprint.nih.gov/ NIH 3D Print Exchange] under the category 'Custom Labware' via our [http://3dprint.nih.gov/users/ethzurichigem2014 ETH_Zurich_iGEM2014] account. | '''All mesh files designed during the project will be made available at the [http://3dprint.nih.gov/ NIH 3D Print Exchange] under the category 'Custom Labware' via our [http://3dprint.nih.gov/users/ethzurichigem2014 ETH_Zurich_iGEM2014] account. | ||
''' | ''' | ||
| + | |||
| + | |||
{{:Team:ETH Zurich/tpl/topbutton|red}} | {{:Team:ETH Zurich/tpl/topbutton|red}} | ||
Revision as of 03:26, 18 October 2014
Millifluidic Chip & Rapid Prototyping
Overview
Our project aims for the biological implementation of cellular automata, so we had to find a way to create a regular grid of cells with a defined neighborhood as shown in the figures below. On the left side a classical cellular automata is depicted (see figure 1), on the right side an outline of our biological version consisting of a grid-like polydimethylsiloxane (PDMS) chip filled with cell colonies encapsulated in alginate beads (see figure 2).
In the following, we have investigated the combination of additive manufacturing (3D-printing) and PDMS chip fabrication for applications in synthetic biology. This rapid prototyping approach allowed us to update our chips continuously according to new insights from modeling or the wet lab and in particular to avoid more intricate photolitographic approaches, which generally require clean room access, relatively expensive raw materials, and in depth knowledge of etching techniques.
As a result, we are convinced that the tinkering with 3D-printing for mold creation is more economical for our applications and measurements. Also it is perfectly in line with the do-it-yourself spirit of iGEM.
Mold Design and 3D Print Exchange
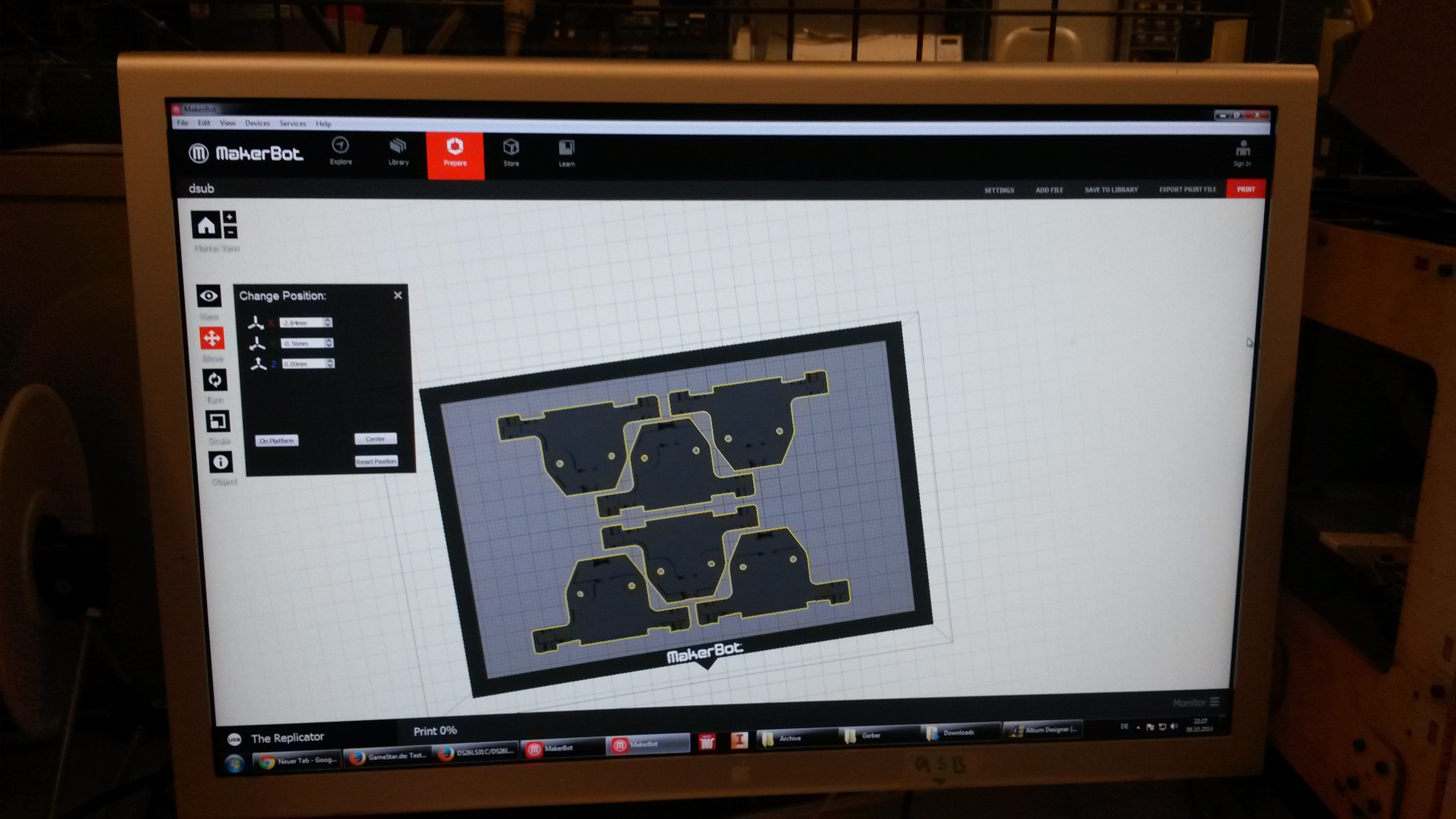
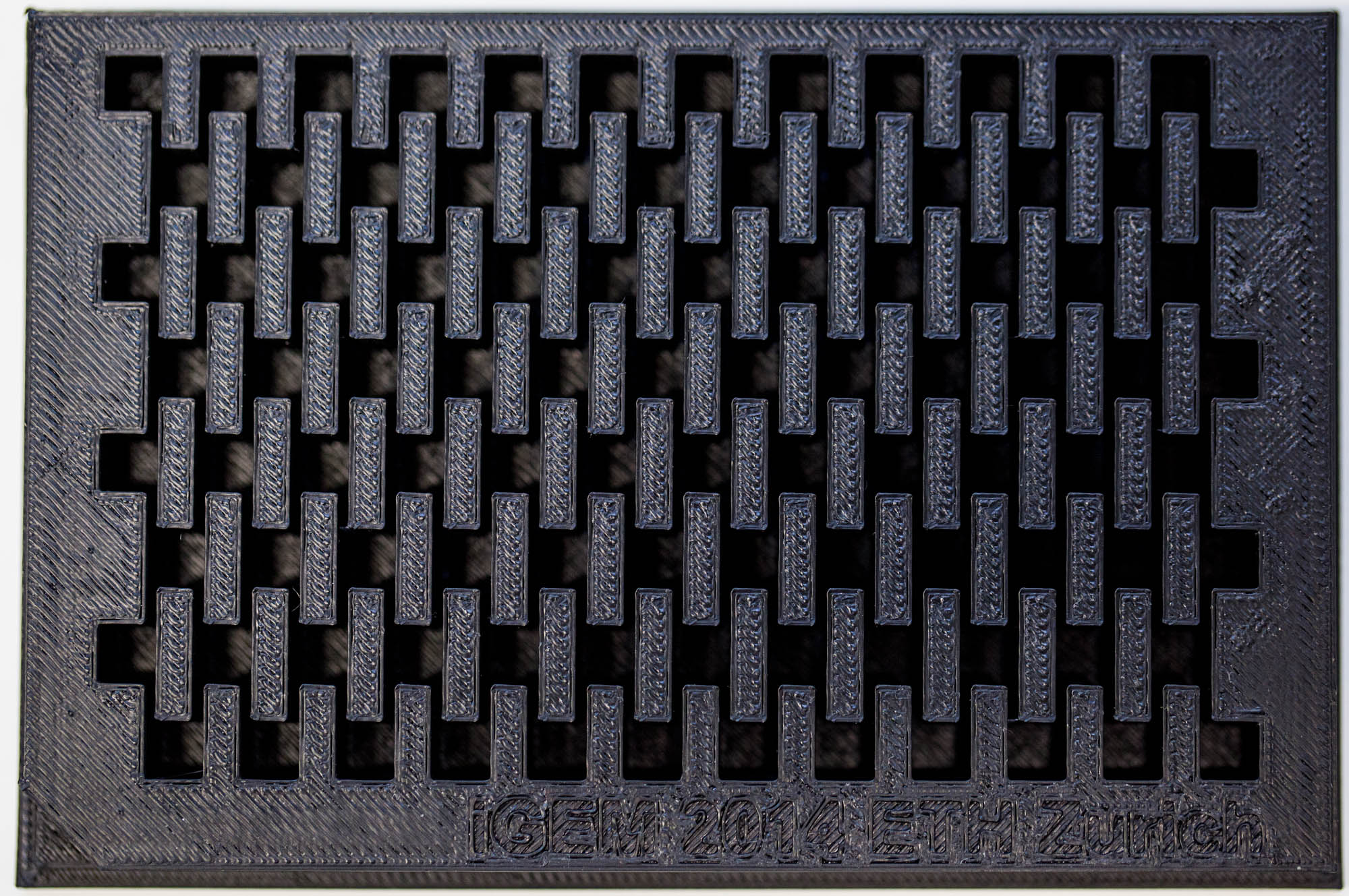
Our custom-made plates and molds were design using a common personal computer (MacBook Air, 13-inch, early 2014, 1.7 GHz Intel Core i7, 8 GB 1600 MHz) and a 3D computer aided design (CAD) software package that is freely available for Mac OS X 10.9.4 ([http://www.123dapp.com/design Autodesk123D Design]). The CAD models were exported as mesh files (.stl) to the 3D printer's software ([http://www.makerbot.com/support/makerware/troubleshooting/ MakerWare]). The dimensions of the device-structures were usually between 1 mm and 5 mm, falling in the range of millifluidics[31].
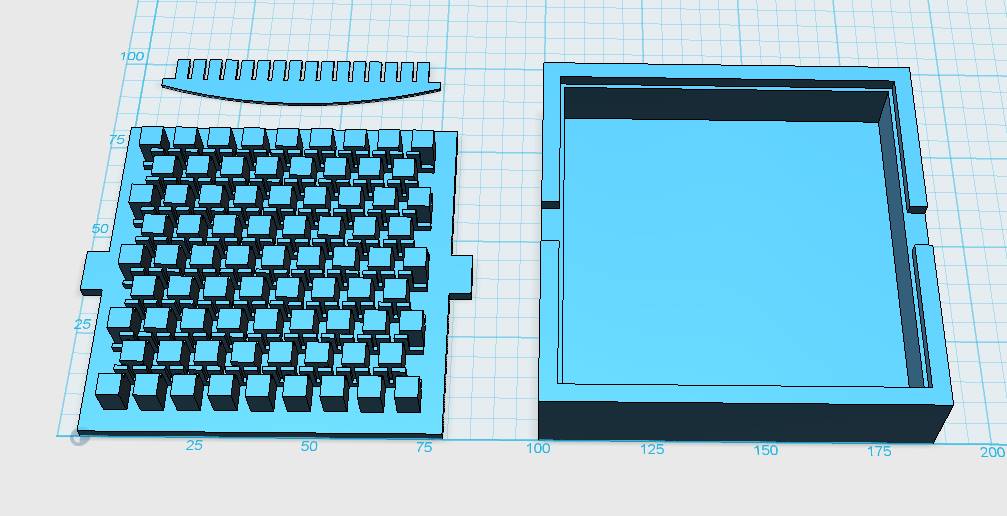
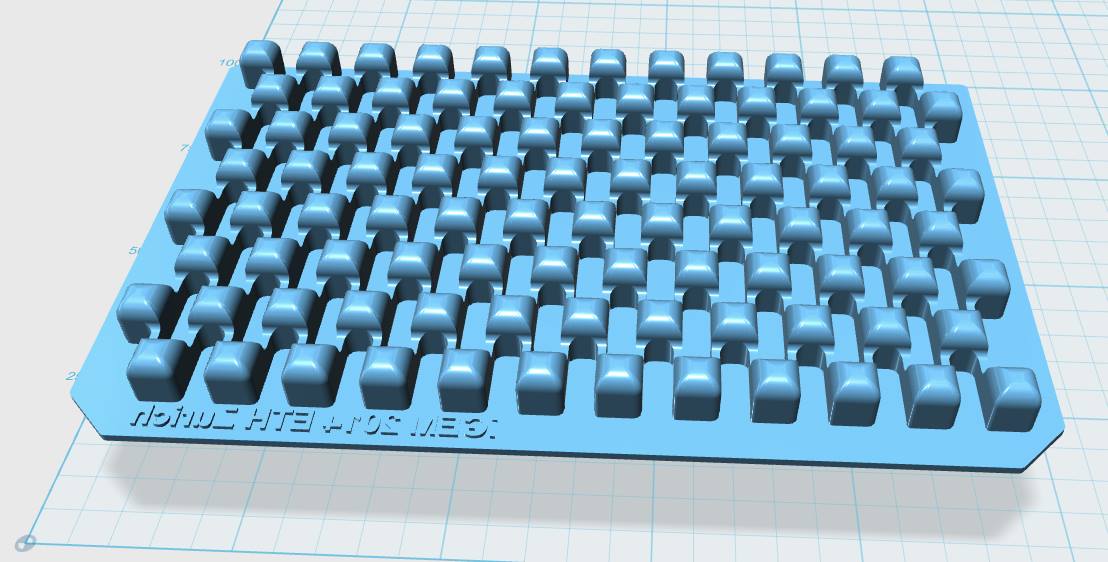
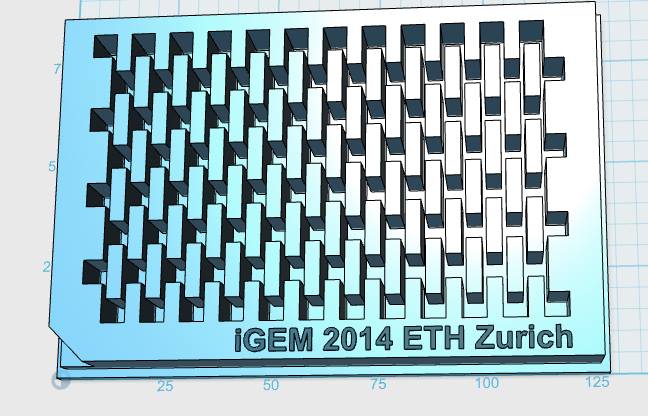
| 
| 
|
| Figure 3-a The first design for a gel-comb, a mold for a millifluidic PDMS chip and a corresponding box for the mold. | Figure 3-b The final mold design for our millifluid PDMS chip used for cell-to-cell communication experiments. | Figure 3-c A design for a 96-well plate with connected wells, which allows automated measurements in a plate reader. |
All mesh files designed during the project will be made available at the [http://3dprint.nih.gov/ NIH 3D Print Exchange] under the category 'Custom Labware' via our [http://3dprint.nih.gov/users/ethzurichigem2014 ETH_Zurich_iGEM2014] account.
3D-Printing and Rapid Prototyping
The mold designs were printed with a commercial 3D-printer (2nd generation MakerBot Replicator with MakerWare software; [http://www.makerbot.com MakerBotIndustries], Brooklyn, US; 5th generation US$2'899) with acrylonitrile butadiene styrene ([http://en.wikipedia.org/wiki/Acrylonitrile_butadiene_styrene ABS], a copolymer of acrylonitrile, butadiene, and styrene). The maximum object size printable is [mm]: 225 x 145 x 150. The precision and minimum feature size are given as [mm]: 0.011 (XY-axis), 0.0025 (Z-axis); and 0.4 (XY-axis), 0.2 (Z-axis) respectively. The printing time varied with the size of the mold but was usually below 4 hours.
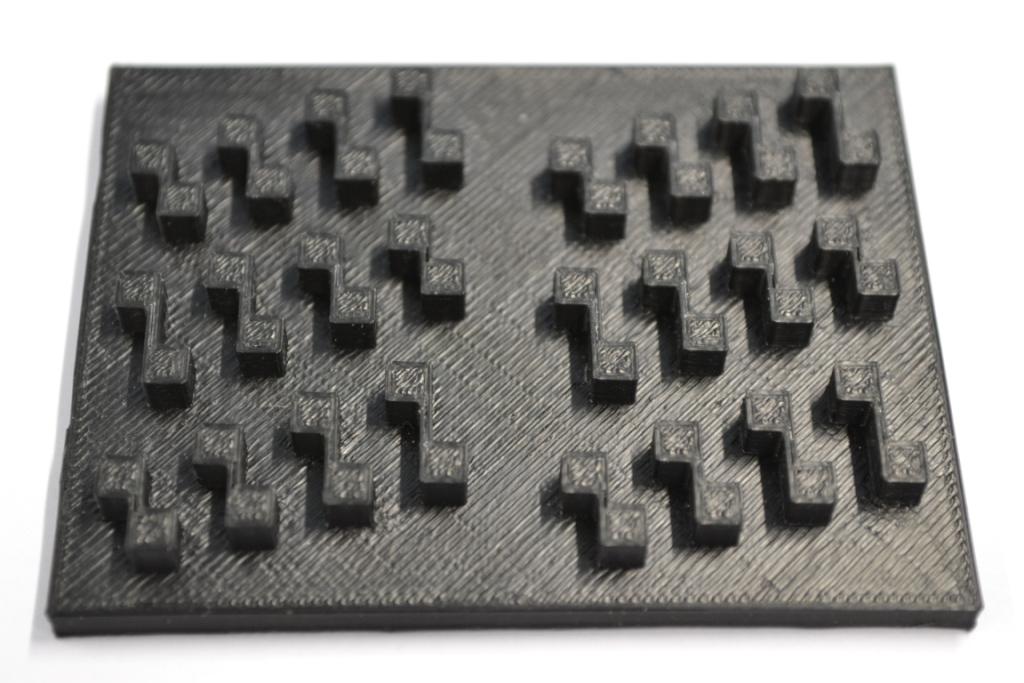
All fabricated structures were ready to use after removing the support structures and did not require additional surface treatments like sonication, curing, painting or silanization. The molds were then directly used for PDMS chip production. In addition, custom made black 96-well plates (connected wells for diffusion assays, plate reader compatible) were printed but found to be leaky over time. The material costs of the molds were in the range of US$2 to US$4 and for the 96-well plates below US$8 (about US$160 per kg of ABS). The maximum resistance to continuous heat is given as 90 ⁰C [23], as a result autoclaving at 121 ⁰C was not feasible and led to deformation (see the box in figure 5-a).
PDMS Chip Preparation
For the fabrication of millifluidic-chips raw PDMS (Dow Corning Sylgard 184) was prepared by mixing base and curing agent in 10:1 proportion. The PDMS solution was mixed vigorously and degassed (desiccator connected to vacuum) until no further bubble formation could be observed. Subsequently the mixture was poured over the mold and cured in an vacuum oven over night at RT. While the first mold design separated insufficiently from the PDMS due to an inappropriate aspect ratio (see figures 6-a and 7-a), all other PDMS chips were easily removed without additional aids and placed in clear plastic trays (86 x 128 mm; OmniTrays, Thermo Scientific). All the mold shown below were at least used once before the pictures were taken. The PDMS wells were then filled with CB medium and loaded with cells encapsulated in alginate beads.

| 
| 
| 
|
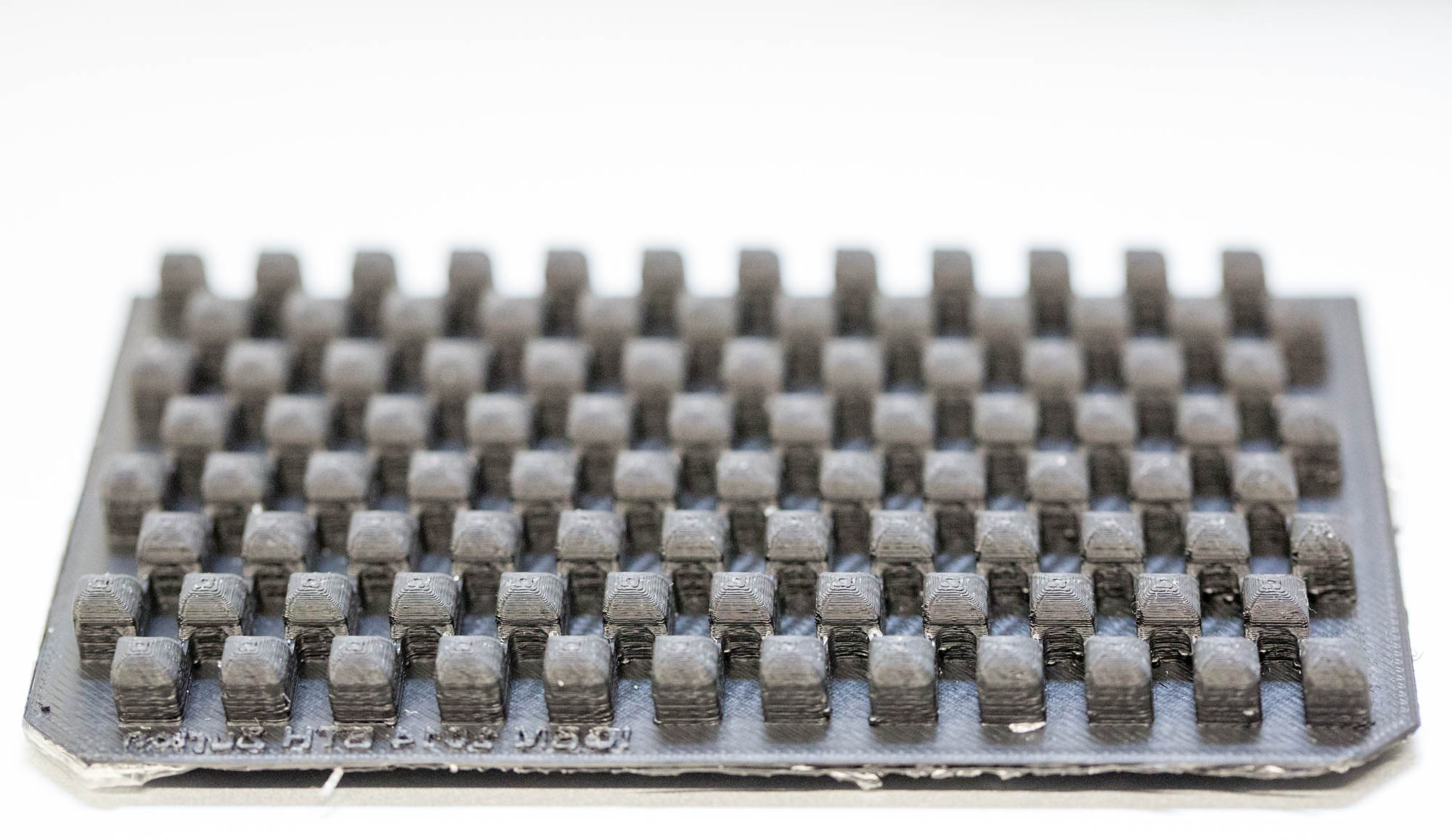
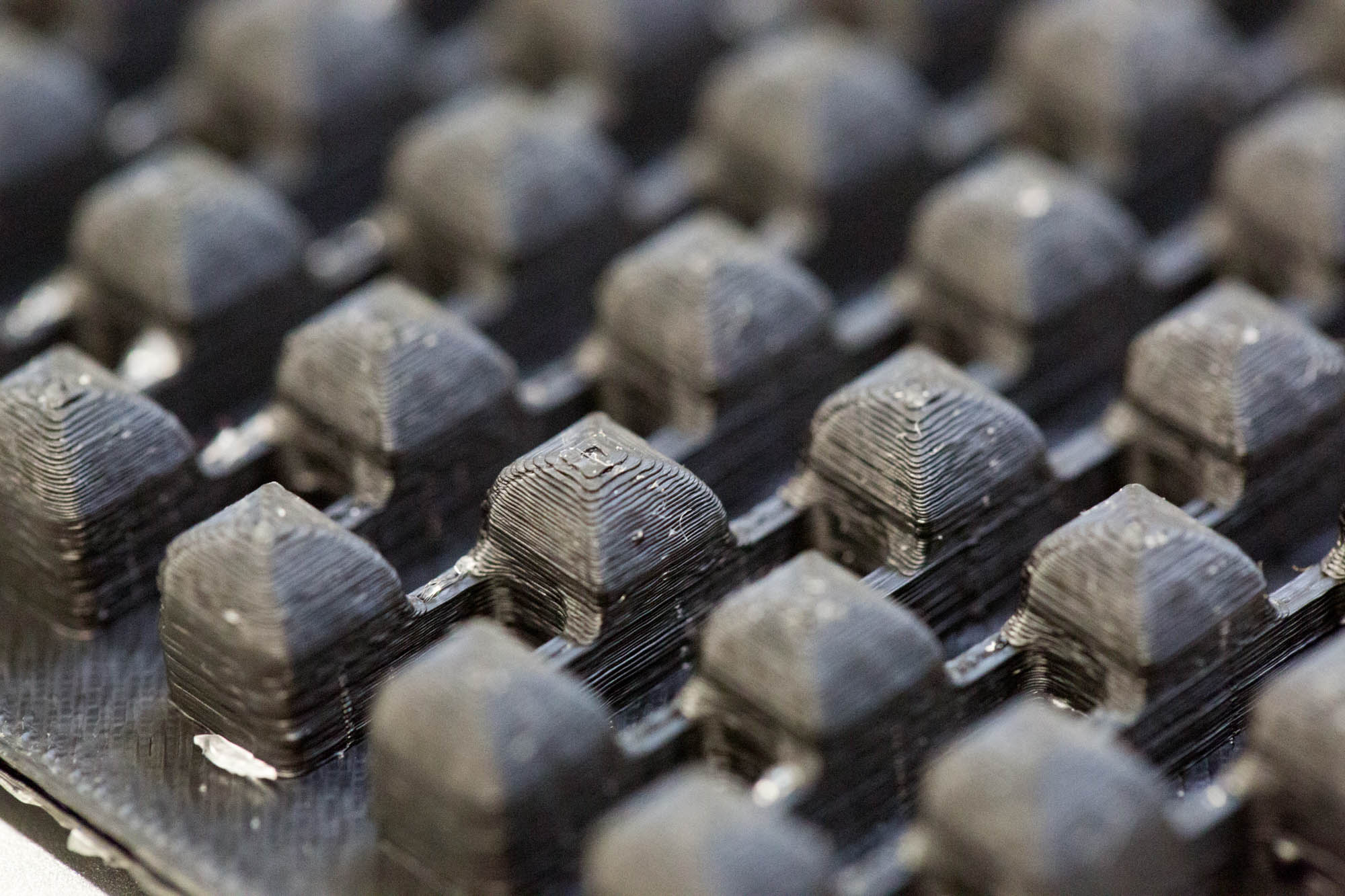
| Figure 6-a The very first mold design. PDMS stuck between the wells while removing it. | Figure 6-b Mold design for a diffusion assay with two connected chambers (edge length of 4 mm) with varied channel length (1 mm to 4 mm). | Figure 6-c The final mold design for cell-to-cell communication experiments (edge length of 5 mm, channel length of 3 mm). | Figure 6-d Close up of the final mold design. The separate layers are clearly visible ('additive' manufacturing). |

| 
| 
| 
|
| Figure 7-a The very first PDMS chip. As the close-up shows, the outer parts are well defined, but the middle part did not separate from the mold due to an inappropriate aspect ratio. | Figure 7-b PDMS chip for diffusion assays with two connected chambers (edge length of 4 mm) and varied channel length (1 mm to 4 mm). | Figure 7-c The final PDMS chip for cell-to-cell communication experiments (edge length of 5 mm, channel length of 3 mm). | Figure 7-d Close up of the final PDMS chip. The channels are well defined and even small structures separated evenly from the mold. |
Time-Lapse Movies
Below you find an overview of the time-lapse movies taken during the summer. In the very first trial the wells were filled with LB agar, holes were punched with a pipette tip and filled with highlighter-ink ([http://en.wikipedia.org/wiki/Pyranine pyranine]) to visualize diffusion (see video 1). Later, different set-ups were tested: chambers filled with liquid LB medium separated by solidified 2% agarose in the connecting channel and alginate beads in liquid CB medium. We continued with the 'alginate beads in liquid medium' set-up, as it yielded the most promising intermediate results, and could then finally show cell-to-cell communication of bacteria confined in beads on our millifluid chip.
In all videos shown imaging was implemented with a Biostep Dark-Hood DH-50 (Argus X1 software) fitted with a Canon EOS 500D DSLR camera and a fluorescence filter (545 nm filter). Pictures were taken every 2 min at an excitation wavelength of 470 nm with the standard Canon EOS Utility software. Time-lapse movies were created with Adobe After Effects CC software.
|
|
|
| Video 1 The very first diffusion experiment with fluorescent highlighter ink ([http://en.wikipedia.org/wiki/Pyranine pyranine]). The wells of the PDMS chip were filled with LB agar. About 5 μL ink were added in a punched whole on one side of the two wells. ~4500x faster than real-time. | Video 2 Diffusion experiment with liquid cultures. The wells of the PDMS chip were filled with LB medium, separated by solidified 2% agarose in the channel. The bottom well contained 3OC6-HSL (~1 mM), the top well E. coli cells with sfGFP under the control of pLux. ~4500x faster than real-time. |
|
|
|
| Video 3 Diffusion experiment with alginate beads in defined liquid medium. The wells of the PDMS chip were filled with CB medium. The bottom well contained beads with 3OC6-HSL (~1 mM), the top well E. coli cells with sfGFP under the control of pLux confined in alginate beads (d=3 mm, intially 107 cell/beads). ~1850x faster than real-time. | Video 4 Cell communication experiment with alginate beads in defined liquid medium. The wells of the PDMS chip were filled with CB medium. The bottom well contained E. coli cells expressing LuxI, which catalyzes the production of 3OC6-HSL; the top well contained E. coli cells with sfGFP under the control of pLux. All cells were confined in alginate beads (d=3 mm, intially 107 cell/beads). ~3450x faster than real-time. |
|
| |
| Video 5 Row wise, self-propagating cell-to-cell communication of E. coli cells confined in alginate beads (d=3 mm, intially 107 cell/beads) on a custom-made millifluidic PDMS chip. All cells contained riboregulated sfGFP followed by [http://parts.igem.org/Part:BBa_C0161 LuxI (BBa_C0161)] together under the control of the [http://parts.igem.org/Part:BBa_R0062 pLux promoter (BBa_R0062)], and [http://parts.igem.org/Part:BBa_J23100 constitutively (BBa_J23100)] expressed [http://parts.igem.org/Part:BBa_C0062 LuxR (BBa_C0062)]. LuxI catalyzes the production of the autoinducer 3OC6-HSL, which is then diffusing from cell to cell. For initialization, the cells in one bead of the top row were induced with 3OC6-HSL before encapsulation. 1750x faster than real-time, the video starts 7 h after the initiation of the experiment. | |
 "
"