|
|
| (18 intermediate revisions not shown) |
| Line 2: |
Line 2: |
| | <html> | | <html> |
| | <div id="main-content"> | | <div id="main-content"> |
| - | <h>Cleanse-Attachment</h> | + | <h>Cleanse-<span style='color:rgb(249, 103, 236);'>Attachment</span></h> |
| | + | <h1>Here’s the Gist…</h1> |
| | <div class='abstract'> | | <div class='abstract'> |
| | <ul> | | <ul> |
| - | <li>To evaluate the efficiency of killing module and antibiofilm module, we develop cohesion part to shorten the geographical distance between S. mutans and E. coli.</li> | + | <li>Help anchor <i>E. coli</i> to the surface of <i>S. mutans</i> for efficient killing and biofilm degradation.</li> |
| - | <li>We use C16, a trunked quorum sensing pheromone as an anchor to attach e-coli to the surface of S. mutans. C16 is displayed on E. coli by INPNC, an E. coli surface display protein.</li>
| + | |
| - | <li>We measure the function of our circuit by three stages, including the function test of INPNC, the display pattern of C16,and the interaction between S. mutans and our modified e-coli.</li>
| + | |
| | </ul> | | </ul> |
| | </div> | | </div> |
| | <div class='cont-panel'> | | <div class='cont-panel'> |
| - | <div href='#2c1-1'><p>purpose</p></div> | + | <div href='#2c1-1'><p>Get started.</p></div> |
| - | <div href='#2c1-2'><p>background</p></div> | + | <div href='#2c1-2'><p>How to do it?</p></div> |
| - | <div href='#2c1-3'><p>design</p></div> | + | <div href='#2c1-3'><p>Test it!</p></div> |
| - | <div href='#2c1-4'><p style="line-height: 25px;">Functional Measurement</p></div> | + | <div href='#2c1-4'><p>Our result~</p></div> |
| - | <div href='#2c1-5'><p>result</p></div> | + | <div href='#2c1-5'><p>Reference</p></div> |
| | <div style="display:inline-block; width: 640px; height: 0.1px; border: none; margin: 0px"></div> | | <div style="display:inline-block; width: 640px; height: 0.1px; border: none; margin: 0px"></div> |
| | </div> | | </div> |
| - | <div class='article'> | + | <div class='article indent'> |
| - | <h1>Purpose</h1> | + | <h1 id='2c1-1'>Before we get started:</h1> |
| - | <p>To facilitate the efficiency of our killing and anti-biofilm parts, we designed a cohesion module. The cohesion module will attach our modified E. coli to the surface of the S. mutans, so we can shorten the distance between our helper E.coli and our targeted enemy, S. mutans. Then, we can evaluate the efficiency of the other mechanism, the “Completion” part in our project.</p>
| + | <p>Although there are a plethora of oral bacteria, only a handful cause tooth decay, and chief among those is <b><i>S. mutans</i> </b>(see <a href='/Team:NYMU-Taipei/project/overview'>[Project Overview]</a> for more information). For our modified <i>E. coli</i> to deal with this threat, we need to find a way for them to <b>get close to <i>S. mutans</i></b> colonies in the oral environment.</p> |
| - | <p>For the Completion-killing part, when the helper E. coli received the signal sent by modified phage, the antibiotic secreted E. coli can kill the S. mutans faster if they are closer to each other. Additionally, E. coli can sense the increase of biofilm formation in a shorter period of time and thus activate the antibioflim module.In conclusion, the “Cohesion” part allows the E. coli to serve as a supervisor around S. mutans and reduces the time and distance of the attacks.</p>
| + | <p>Competence Stimulating Peptide, or <b>CSP</b>, is an important <b>quorum sensing (QS) pheromone specific to <i>S. mutans</i></b>, playing a huge role in their genetic response to population density. The system works since <i>S. mutans</i> continuously release CSP, which <b>bind to the surface receptors</b> of other <i>S. mutans</i> nearby. Check out <a href='/Team:NYMU-Taipei/project/1c'>[Control: Target]</a> for more information on CSP. </p> |
| - | <h1>Background</h1> | + | |
| - | <p>In order to make E. coli a supervisor of the population of S. mutans, we have to find an anchor to attach E. coli to S. mutans. Thus, we take advantage of the CSP. CSP is a kind of quorum sensing pheromone, which enables Streptococcus mutans to alter their gene expression when the critical density of cell population is reached. Recent studies showed that density-dependent quorum sensing (QS) system is primarily comprised of the Competence Stimulating Peptide (CSP) and the ComD/ComE two-component signal transduction system. In addition to biofilm formation, the CSP-mediated QS system in S. mutans also affects its acidogenicity, aciduricity, genetic transformation and bacteriocin production. Most importantly,CSP is specificly secreted by S. mutans, which is the reason why we chose it as anchor targeting S. mutans.</p>
| + | <p>The exclusivity of CSP and the ubiquity of its receptors in <i>S. mutans</i> makes it a great candidate for creating an anchoring module! </p> |
| - | <h1>Design</h1>
| + | |
| - | <p>We use competence stimulating peptide (CSP) as a targeting material to attach E. coli to the surface of Streptococcus mutans. To do so, we utilize Surface display protein INPNC (designed by <a href="https://2011.igem.org/Team:Edinburgh" target="_blank">2011 iGEM Edinburgh team</a>) to display CSP on the membrane of E. coli, which act as the “anchor”. CSP binds to both the receptors on the surface of Streptococcus mutans and our engineered helper E. coli.</p>
| + | <h1 id='2c1-2'>So how did we do it?</h1> |
| - | <p>!fig1 not yet!</p>
| + | <p>Because of size constraints, we did not want to use the actual CSP molecule itself. Instead, we found <b>C16</b>, a functionally active <b>fragment of CSP</b> which still binds to CSP receptors. Compared to CSP, C16 is smaller and more suitable for attachment on the <i>E. coli</i> surface. </p> |
| - | <p>!circuit fig not yet!</p>
| + | |
| - | <h1>Functional Measurement</h1>
| + | <p>Now that the attachment candidate is found, what’s left is to express it on the surface of <i>E. coli</i>, so it can interact with <i>S. mutans</i>.</p> |
| - | <h3>Working circuit:</h3>
| + | |
| - | <img src=''><p>(圖1)</p>
| + | <p>For that purpose, we found <b>INPNC</b>, a <b>surface display protein</b> in <i>E. coli</i> biobricked by the 2011 Edinburgh iGEM team. Using INPNC as basis, we created the INPNC-C16 fusion protein, which displays C16 on the surface of <i>E. coli</i> to achieve the results we want. </p> |
| - | <br>
| + | |
| - | <h3>Testing circuit:</h3>
| + | <p>At last, our circuit is complete! </p> |
| - | <img src=''><p>(圖2)</p>
| + | <img src="/wiki/images/7/7d/NYMU14_attach_Attachment.png" style='width:600px;'> |
| - | <h3>Method:</h3>
| + | <p>If everything works as planned, this circuit should express C16 on the surface of <i>E. coli</i> via INPNC, which will then bind to CSP receptors on <i>S. mutans</i>, anchoring the two cells together.</p> |
| - | <p>Use 5cc LB culture and 5 ul chloramphenicol antibiotic to incubate bacteria transformed by function test circuit( K523013 + CSP16 + E1010 and B0015)and control circuit (K523013 + CSP16 + and B0015 )12~16 hour. Then centrifuge 5cc bacteria culture at 13000 rps for 2 minute.</p>
| + | <img src='/wiki/images/5/5a/NYMU14_attach_mech.png' style='width: 280px;margin-left: 40px;'> |
| - | <img src=''><p>(圖3)</p>
| + | |
| - | <h1>Result</h1> | + | <h1 id='2c1-3'>Putting it to the test!</h1> |
| - | <img src=''><p>(圖4)</p>
| + | <p>Having a circuit is only the start! Now we must test how well our circuits function, specifically if INPNC can truly anchor the fusion protein on the cell membrane. The first test is a qualitative assay to determine if the fusion protein can truly function as intended, and the second is a quantitative test to further reinforce the conclusions of the first.</p> |
| - | <h1>Reference</h1> | + | <h3>Is the INPNC-C16 fusion protein on the membrane?</h3> |
| - | <ol>
| + | <p>To test whether or not our INPNC-C16 fusion protein can be successfully expressed on the surface of our <i>E. coli</i>, we constructed a testing circuit with RFP after C16:</p> |
| - | <li>Targeted Killing of Streptococcus mutans by a Pheromone-Guided “Smart” Antimicrobial Peptide(2006)Randal Eckert1, Jian He2, Daniel K. Yarbrough2, Fengxia Qi2,Maxwell H. Anderson3 and Wenyuan Shi</li>
| + | <img src='/wiki/images/a/af/NYMU14_attach_AttachmentT1.png' style='width: 630px;'> |
| - | <li>Quorum sensing and biofilm formation by Streptococcus mutans. Senadheera.(2008) D1, Cvitkovitch DG</li>
| + | <p>We expect this circuit to produce an INPNC-C16-RFP fusion protein with RFP on the very outer side of the membrane, shown below.</p> |
| - | </ol>
| + | <img src='/wiki/images/d/d0/NYMU14_attach_Working1.png' style='width: 170px;margin-left: 40px;'> |
| - | </div> <!-- article-->
| + | <p>At the same time, we used our functional circuit design as a control:</p> |
| | + | <img src='/wiki/images/7/7d/NYMU14_attach_Attachment.png' style='width: 570px;'> |
| | + | <p>We expect this circuit to produce everything identically, besides RFP, and that it will produce a colorless tube.</p> |
| | + | <img src='/wiki/images/9/93/NYMU14_attach_tube1.png' style='width: 200px;margin-left: 40px;'> |
| | + | <p>After constructing our testing circuit, we incubated the transformed bacteria together for 12 ~ 16 hours, before centrifugation. Theoretically, there are three possible results for our testing.</p> |
| | + | |
| | + | <h3><u>Possible Result 1: Fusion protein is on cell membrane</u></h3> |
| | + | <p>This is our anticipated result. If the fusion protein is on the membrane, then after centrifuge the pellet should display a <b>very clear red color</b>.</p> |
| | + | <img src='/wiki/images/a/a5/NYMU14_attach_tube_res1.png' style='width: 220px;margin-left: 40px;'> |
| | + | <h3><u>Possible Result 2: Fusion protein is inside cell membrane</u></h3> |
| | + | <p>If for some reason the fusion protein failed to display on the cell membrane, we will see a <b>dull whitish-red color</b> after centrifuge.</p> |
| | + | <img src='/wiki/images/2/22/NYMU14_attach_tube_res2.png' style='width: 220px;margin-left: 40px;'> |
| | + | <h3><u>Possible Result 3: Fusion protein is outside cell membrane</u></h3> |
| | + | <p>If for some reason the fusion protein had been exported outside the cell, we will see a <b>dull red color in the supernatant</b>.</p> |
| | + | <img src='/wiki/images/3/3d/NYMU14_attach_tube_res3.png' style='width: 230px;margin-left: 40px;'> |
| | + | |
| | + | <h3>Here is the details of our method</h3> |
| | + | <ol> |
| | + | <li>Use 5ml LB culture and 5μl chloramphenicol antibiotic to incubate bacteria transformed by function test circuit (K523013 + CSP16 + E1010 and B0015) and control circuit (K523013 + CSP16 + and B0015) 12~16 hour.</li> |
| | + | <li>Centrifuge 5cc bacteria culture at 13000 rps for 2 minute.</li> |
| | + | </ol> |
| | + | <p>Our result turn out to be a clear red color! This indicates that our fusion protein has been successfully displayed on the surface of the cell membrane.</p> |
| | + | |
| | + | <h3>Testing the display function and efficiency of INPNC</h3> |
| | + | <p>We designed the experiment below.</p> |
| | + | <ol> |
| | + | <li>Cultured our modified e-coli containing test circuit(promoter+INPNC+CSP16+RFP+terminator) in LB liquid culture for 12 hours.</li> |
| | + | <li>Used 96 well plate to culture above-mentioned e-coli.</li> |
| | + | <li>Used varioskan flash spectral scanning multimode reader to test fluorescence curve and OD curve every 2 hours.</li> |
| | + | <li>Also, compared culture in LB culture and PBS culture.</li> |
| | + | <li>Meanwhile, LB culture +competence cell and PBS culture+ competence cell as control.</li> |
| | + | </ol> |
| | + | <img src='/wiki/images/d/de/NYMU14_attach_well.png' style='width: 600px;'> |
| | + | |
| | + | <h1 id='2c1-4'>Our results</h1> |
| | + | <h3>First test</h3> |
| | + | <p>After centrifugation, the supernatant is clear; we could indicate that CSP16 is not secreted out of cell. Also, indicated from the red fluorescence of the pellet, we could say that INPNC protein display CSP16 and RFP on the surface.</p> |
| | + | <img class='bdframe' src='/wiki/images/c/cb/NYMU14_attach_first_res.png' style='width: 500px;'> |
| | + | |
| | + | <h3>Second test</h3> |
| | + | <p>At the beginning, the population of <i>E. coli</i> is small as well as OD, so initial figure (Fluorescence /OD) is large. As time proceeds, Fluorescence /OD level increases dramatically, indicating that the INPNC protein is being greatly expressed. The OD level reaches a plateau at the end.</p> |
| | + | <img src='/wiki/images/c/c4/NYMU14_attach_second_res.png' style='width:500px;'> |
| | + | <p>Fluorescence level /OD level indicates the display function of INPNC through growth time.</p> |
| | + | <img src="/wiki/images/3/37/NYMU14_attach_second_res2.PNG"> |
| | + | |
| | + | </div> <!-- article --> |
| | + | <h1 id='2c1-5'>Reference</h1> |
| | + | <ol> |
| | + | <li>Na, D., Yoo, S. M., Chung, H., Park, H., Park, J. H., & Lee, S. Y. (January 01, 2013). Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nature Biotechnology, 31, 2, 170-4.</li> |
| | + | <li>Li, Y.-H., Lau, P. C. Y., Tang, N., Svensater, G., Ellen, R. P., & Cvitkovitch, D. G. (November 15, 2002). Novel Two-Component Regulatory System Involved in Biofilm Formation and Acid Resistance in Streptococcus mutans. Journal of Bacteriology, 184, 22, 6333-6342.</li> |
| | + | <li>Baev, D., England, R., & Kuramitsu, H. K. (January 01, 1999). Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy. Infection and Immunity, 67, 9, 4510-6.</li> |
| | + | <li>Yoshida, A., & Kuramitsu, H. K. (December 01, 2002). Multiple Streptococcus mutans Genes Are Involved in Biofilm Formation. Applied and Environmental Microbiology, 68, 12, 6283-6291.</li> |
| | + | <li>Biswas, I., Jha, J. K., & Fromm, N. (August 01, 2008). Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology, 154, 8, 2275-2282.</li> |
| | + | <li>Li, Yung-Hua, Tang, Nan, Aspiras, Marcelo B., Lau, Peter C. Y., Lee, Janet H., Ellen, Richard P., & Cvitkovitch, Dennis G. (n.d.). A Quorum-Sensing Signaling System Essential for Genetic Competence in Streptococcus mutans Is Involved in Biofilm Formation. American Society for Microbiology.</li> |
| | + | <li>Wu, J., Cho, M. I., & Kuramitsu, H. K. (January 01, 1995). Expression, purification, and characterization of a novel G protein, SGP, from Streptococcus mutans. Infection and Immunity, 63, 7, 2516-21.</li> |
| | + | <li>Targeted Killing of Streptococcus mutans by a Pheromone-Guided “Smart” Antimicrobial Peptide(2006)Randal Eckert1, Jian He2, Daniel K. Yarbrough2, Fengxia Qi2,Maxwell H. Anderson3 and Wenyuan Shi</li> |
| | + | <li>Quorum sensing and biofilm formation by Streptococcus mutans. Senadheera.(2008) D1, Cvitkovitch DG</li> |
| | + | </ol> |
| | </div> | | </div> |
| | </html> | | </html> |
| | {{:Team:NYMU-Taipei/NYMU14_Footer}} | | {{:Team:NYMU-Taipei/NYMU14_Footer}} |
Cleanse-Attachment
Here’s the Gist…
- Help anchor E. coli to the surface of S. mutans for efficient killing and biofilm degradation.
Before we get started:
Although there are a plethora of oral bacteria, only a handful cause tooth decay, and chief among those is S. mutans (see [Project Overview] for more information). For our modified E. coli to deal with this threat, we need to find a way for them to get close to S. mutans colonies in the oral environment.
Competence Stimulating Peptide, or CSP, is an important quorum sensing (QS) pheromone specific to S. mutans, playing a huge role in their genetic response to population density. The system works since S. mutans continuously release CSP, which bind to the surface receptors of other S. mutans nearby. Check out [Control: Target] for more information on CSP.
The exclusivity of CSP and the ubiquity of its receptors in S. mutans makes it a great candidate for creating an anchoring module!
So how did we do it?
Because of size constraints, we did not want to use the actual CSP molecule itself. Instead, we found C16, a functionally active fragment of CSP which still binds to CSP receptors. Compared to CSP, C16 is smaller and more suitable for attachment on the E. coli surface.
Now that the attachment candidate is found, what’s left is to express it on the surface of E. coli, so it can interact with S. mutans.
For that purpose, we found INPNC, a surface display protein in E. coli biobricked by the 2011 Edinburgh iGEM team. Using INPNC as basis, we created the INPNC-C16 fusion protein, which displays C16 on the surface of E. coli to achieve the results we want.
At last, our circuit is complete!

If everything works as planned, this circuit should express C16 on the surface of E. coli via INPNC, which will then bind to CSP receptors on S. mutans, anchoring the two cells together.

Putting it to the test!
Having a circuit is only the start! Now we must test how well our circuits function, specifically if INPNC can truly anchor the fusion protein on the cell membrane. The first test is a qualitative assay to determine if the fusion protein can truly function as intended, and the second is a quantitative test to further reinforce the conclusions of the first.
Is the INPNC-C16 fusion protein on the membrane?
To test whether or not our INPNC-C16 fusion protein can be successfully expressed on the surface of our E. coli, we constructed a testing circuit with RFP after C16:

We expect this circuit to produce an INPNC-C16-RFP fusion protein with RFP on the very outer side of the membrane, shown below.
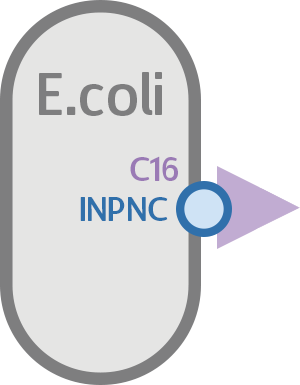
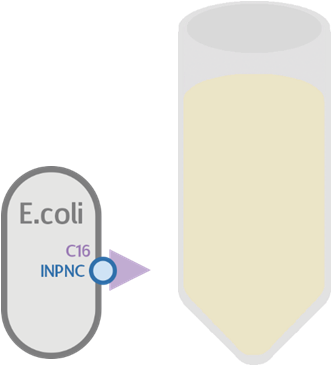
At the same time, we used our functional circuit design as a control:

We expect this circuit to produce everything identically, besides RFP, and that it will produce a colorless tube.
After constructing our testing circuit, we incubated the transformed bacteria together for 12 ~ 16 hours, before centrifugation. Theoretically, there are three possible results for our testing.
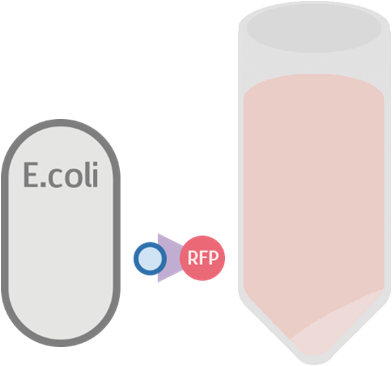
Possible Result 1: Fusion protein is on cell membrane
This is our anticipated result. If the fusion protein is on the membrane, then after centrifuge the pellet should display a very clear red color.
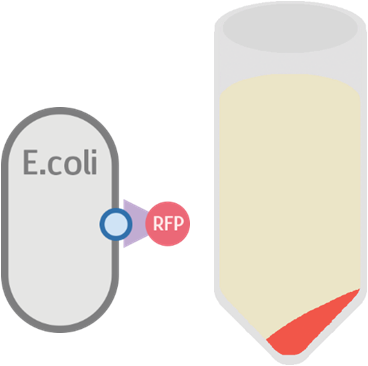
Possible Result 2: Fusion protein is inside cell membrane
If for some reason the fusion protein failed to display on the cell membrane, we will see a dull whitish-red color after centrifuge.
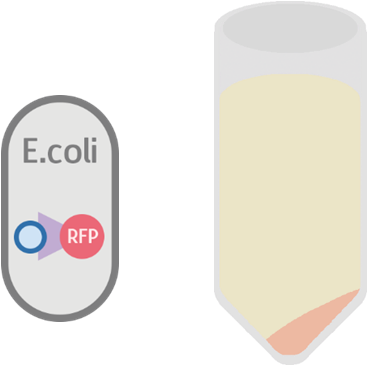
Possible Result 3: Fusion protein is outside cell membrane
If for some reason the fusion protein had been exported outside the cell, we will see a dull red color in the supernatant.
Here is the details of our method
- Use 5ml LB culture and 5μl chloramphenicol antibiotic to incubate bacteria transformed by function test circuit (K523013 + CSP16 + E1010 and B0015) and control circuit (K523013 + CSP16 + and B0015) 12~16 hour.
- Centrifuge 5cc bacteria culture at 13000 rps for 2 minute.
Our result turn out to be a clear red color! This indicates that our fusion protein has been successfully displayed on the surface of the cell membrane.
Testing the display function and efficiency of INPNC
We designed the experiment below.
- Cultured our modified e-coli containing test circuit(promoter+INPNC+CSP16+RFP+terminator) in LB liquid culture for 12 hours.
- Used 96 well plate to culture above-mentioned e-coli.
- Used varioskan flash spectral scanning multimode reader to test fluorescence curve and OD curve every 2 hours.
- Also, compared culture in LB culture and PBS culture.
- Meanwhile, LB culture +competence cell and PBS culture+ competence cell as control.
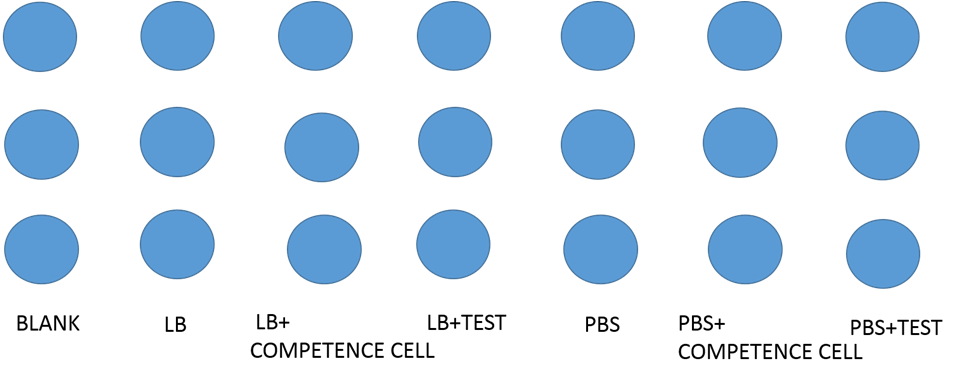
Our results
First test
After centrifugation, the supernatant is clear; we could indicate that CSP16 is not secreted out of cell. Also, indicated from the red fluorescence of the pellet, we could say that INPNC protein display CSP16 and RFP on the surface.

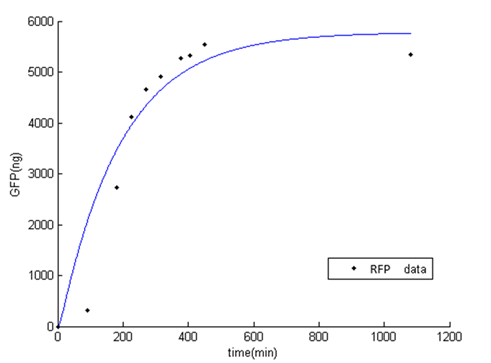
Second test
At the beginning, the population of E. coli is small as well as OD, so initial figure (Fluorescence /OD) is large. As time proceeds, Fluorescence /OD level increases dramatically, indicating that the INPNC protein is being greatly expressed. The OD level reaches a plateau at the end.
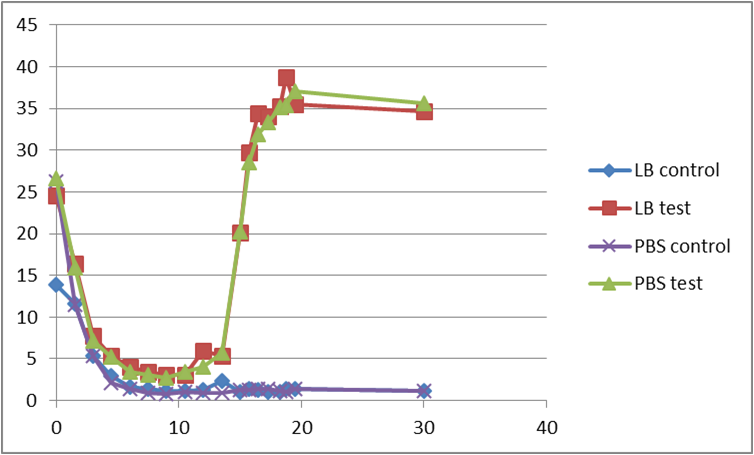
Fluorescence level /OD level indicates the display function of INPNC through growth time.
Reference
- Na, D., Yoo, S. M., Chung, H., Park, H., Park, J. H., & Lee, S. Y. (January 01, 2013). Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nature Biotechnology, 31, 2, 170-4.
- Li, Y.-H., Lau, P. C. Y., Tang, N., Svensater, G., Ellen, R. P., & Cvitkovitch, D. G. (November 15, 2002). Novel Two-Component Regulatory System Involved in Biofilm Formation and Acid Resistance in Streptococcus mutans. Journal of Bacteriology, 184, 22, 6333-6342.
- Baev, D., England, R., & Kuramitsu, H. K. (January 01, 1999). Stress-induced membrane association of the Streptococcus mutans GTP-binding protein, an essential G protein, and investigation of its physiological role by utilizing an antisense RNA strategy. Infection and Immunity, 67, 9, 4510-6.
- Yoshida, A., & Kuramitsu, H. K. (December 01, 2002). Multiple Streptococcus mutans Genes Are Involved in Biofilm Formation. Applied and Environmental Microbiology, 68, 12, 6283-6291.
- Biswas, I., Jha, J. K., & Fromm, N. (August 01, 2008). Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology, 154, 8, 2275-2282.
- Li, Yung-Hua, Tang, Nan, Aspiras, Marcelo B., Lau, Peter C. Y., Lee, Janet H., Ellen, Richard P., & Cvitkovitch, Dennis G. (n.d.). A Quorum-Sensing Signaling System Essential for Genetic Competence in Streptococcus mutans Is Involved in Biofilm Formation. American Society for Microbiology.
- Wu, J., Cho, M. I., & Kuramitsu, H. K. (January 01, 1995). Expression, purification, and characterization of a novel G protein, SGP, from Streptococcus mutans. Infection and Immunity, 63, 7, 2516-21.
- Targeted Killing of Streptococcus mutans by a Pheromone-Guided “Smart” Antimicrobial Peptide(2006)Randal Eckert1, Jian He2, Daniel K. Yarbrough2, Fengxia Qi2,Maxwell H. Anderson3 and Wenyuan Shi
- Quorum sensing and biofilm formation by Streptococcus mutans. Senadheera.(2008) D1, Cvitkovitch DG













 "
"