Team:Heidelberg/pages/PCR 2.0
From 2014.igem.org
(→Introduction) |
|||
| Line 1: | Line 1: | ||
=Introduction= | =Introduction= | ||
| - | + | ==Motivation== | |
| - | + | ||
| - | + | The invention of the polymerase chain reaction in 1983 by Kary Mulliy revolutionized the modern world by enabling amplification of DNA in an exponential manner. Further improvements including the use of thermo-stable DNA-polymerases made the method even more efficient, allowing its widespread use in nearly every field of modern diagnostics and research. However a major part of information is lost when using conventional PCR due to missing transfer of DNA modifications. Those covalently attached functional groups are present all kingdoms of life and act as prominent regulators of gene expression, adding an additional level of complexity. | |
| - | + | We propose the PCR 2.0 as an easy and efficient way to amplify DNA templates in an exponential manner while maintaining their specific methylation pattern. The pivotal element of this PCR reaction is the use of a heat stable DNA methyltransferase (DNMT). Although comparable enzymes exist in thermophile organisms [[#References|[1]]] until now no suitable protein could be found or synthesized that withstands the harsh conditions of a PCR. | |
| - | + | Even though the detection and mapping of methylation patterns has become feasible in a high-throughput manner by using bisulfite sequencing and array techniques [[#References|[2]]], further functional analysis is still limited due to the small amount of primary material. Our approach therefore aims to provide unlimited amounts of any methylated DNA sample of interest, withouth having to know its actual structure or the need of expensive methods and de-novo synthesis – just by running a PCR. | |
| - | + | Hence, main goal of our project has been the generation of a heat stable Dnmt that can be produced in a large scale and shows efficient and specific methylation of DNA. Our approach applies protein circularization by using self-splicing protein sequences, socalled inteins from our toolbox as well as our linker design software (links). Since the restriction of conformational changes through intramolecular bonds has been known to increase the stability of proteins (Vieille, C. & Zeikus, G. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65, 1–43 (2001); Antos, J. M., Popp, M. W.-L., Ernst, R., Chew, G.-L., Spooner, E., & Ploegh, H. L. (2009). A straight path to circular proteins. The Journal of Biological Chemistry, 284(23), 16028–36.) | |
| + | and joining of C and N terminus were reported to increases in thermostability of smaller peptides (Tam, J. P. & Wong, C. T. T. Chemical synthesis of circular proteins. J. Biol. Chem. 287, 27020–5 (2012) references xylanase gfp…) we started the circularization of the so far biggest protein… | ||
| + | ==Epigenetics - there is more than just ATC and G…== | ||
| + | In mammals and other vertebrates the methylation of cytosine nucleotides that are followed by guanines (CpG) is a prominent key regulator of transcription, embryonic development, X chromosome inactivation and many other cellular functions. (Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005); Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 Suppl, 245–54 (2003).). Approximately 1% of the genetic code in human somatic cells is consisting of methylated cytosines, amounting to 70-80% of all present CpG Dinucleotides. | ||
| + | (Ehrlich, M. 1982. Amount and distribution of 5-methycytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 10: 2709–2721.) Due to this great prevalence and the heredity of this modification, the 5-methylcytosine (5mC) is also known as the “fifth base” of eukaryotic genomes. (Lister, R. & Ecker, J. Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome Res. 959–966 (2009)). DNA methylation is preferentially occurring at intergenic regions and repetitive sequences, where it is known to silence gene expression (Bird, A. (2002). DNA methylation patterns and epigenetic memory. Genes & Development 16, 6-21.); Jaenisch, R., and Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33, 245-254.) As against that, GC-rich promoter regions are usually characterized by a low methylation status, allowing transcription of the associated genes (Weichenhan, D., and Plass, C. (2013). The evolving epigenome. Human Molecular Genetics 22, R1. Disregulations of Cytosine methylation have been reported to play a crucial role in the development of numerous diseases, including cancer, imprinting diseases and repeat-instability diseases including morbus huntington (Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005)) | ||
| + | |||
| + | ==A closer look at DNA Methyltransferases== | ||
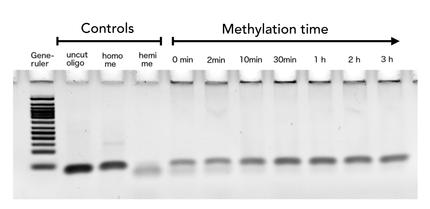
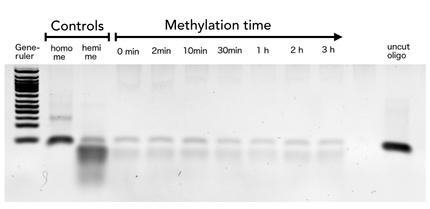
| - | + | The family of enzymes called DNA methyltransferases (DNMTs) is responsible for the establishment and maintenance of most cell-type specific DNA methylation patterns. Whereas the two enzymes DNMT3a and DNMT3b contribute to de novo methylation of DNA during development, DNMT1 is responsible to preserve existing methylation patterns throughout cell divisions. Therefore DNMT1 exploits the principle of semiconservative replication, using the parental strand as a template to create an exact copy of methylation patterns. Subsequent to recognition of a socalled hemi-methylated CpG site, the enzymatic machinery transferres a methyl group from the methyl donor S-Adeosyl-methionine (SAM) to the C5 position of the complementary cytosine residue. (Jurkowska, R. Z., Jurkowski, T. P. & Jeltsch, A. Structure and function of mammalian DNA methyltransferases. Chembiochem 12, 206–22 (2011).) | |
| - | + | ||
| - | + | ||
| - | + | ||
{{:Team:Heidelberg/templates/image-half| | {{:Team:Heidelberg/templates/image-half| | ||
align=right| | align=right| | ||
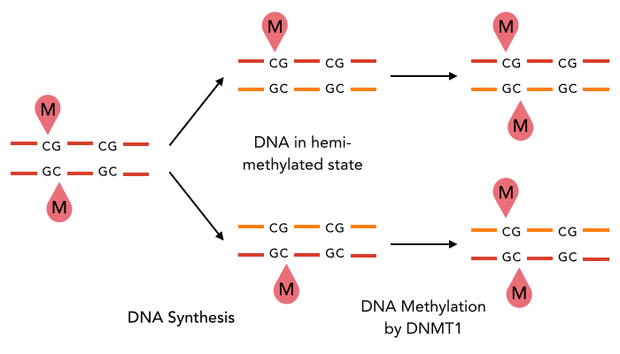
caption=Figure 1) DNMT1 maintenance methylation| | caption=Figure 1) DNMT1 maintenance methylation| | ||
| - | descr= | + | descr=The enzyme DNMT1 is of particular interest for methylation maintenance PCR, as it allows exact duplication of the original methylation pattern to every new DNA copy.| |
file=methylation_principle.png}} | file=methylation_principle.png}} | ||
| - | + | For our experiments we used the smallest truncated version of DNMT1 that has been reported to efficiently mediate methylation maintenance. This truncated version derives from <i>Mus musculus </i> and comprises amino acids 731-1602 (mDNMT1(731-1602) Song, J., Teplova, M., Ishibe-Murakami, S. & Patel, D. J. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science 335, 709–12 (2012).; Bashtrykov, P. et al. Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem. Biol. 19, 572–8 (2012)) kindly provided by Dr. Bashtrykov after approval). mDNMT1(731-1602) contains the C-terminal catalytic methyltransferasedomain as well as the aligning bromo-adjacent homology (BAH) domains, which prevent the binding of unmethylated DNA in the catalytic core. The enzyme is missing the CXXC domain, which has a high affinity to CpG hemi-methylated dinucleotides, but was shown to be dispensable for protein function (Bashtrykov, P., Jankevicius, G., Smarandache, A., Jurkowska, R. Z., Ragozin, S., & Jeltsch, A. (2012). Specificity of DNMT1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chemistry & Biology, 19(5), 572–8.). The truncated version has similar methylation activity and specificity as the wild type, as shown by our own experiments and by Bashtrykov and colleagues. | |
| - | + | In contrast to the full-length DNMT1 that can only be expressed in insect cells using a baculovirus based system, the mDNMT1(731-1602) has the great advantage of being efficiently expressed in <i>E.coli</i>. Since the N and C terminal ends of DNMT1 (731-1602) are closer together than in the full-lengh DNMT1, cirularisation might cause less deformation and have a lower impact on the overall activity, making this truncated mDNMT1 an ideal target for our purposes. (Figure: cristal structure) | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
{{:Team:Heidelberg/templates/image-half| | {{:Team:Heidelberg/templates/image-half| | ||
| Line 42: | Line 35: | ||
file=dnmt1_structure.png}} | file=dnmt1_structure.png}} | ||
| - | However, even the truncated | + | ==Circularization - The missing link== |
| - | We suspected circularization by direct fusion of both termini might cause | + | |
| - | + | However, even the truncated DNMT1 is a very large protein of 100kDa and consists of 872 amino acids. To our knowledge, a protein this size has never been circularized before. According to the crystal structure, the distance between the termini of the truncated DNMT1 is 48 Å. We suspected that circularization of the protein by direct fusion of both termini might cause deformation of the protein structure. Therefore we generated a linker amino acid sequence, adapted to the structure of DNMT1: it needed to be long enough to bridge the gap of 48 Å and should bypass the catalytic core located prominently in the middle of the enzyme structure. | |
| - | + | Since we were not able to estimate the impact of the linker introduction, we focused on two different kinds of linkers: a rather rigid linker that has been calculated and optimized during the establishment of our linker and a flexible peptide connection consisting mostly of Glycine and Serine amino acids. | |
| + | To perform circularization we have used the split NpuDnaE Intein since it is known as the <i> golden standard </i> of protein splicing and has been efficiently tested by us through circularization of GFP, Lysosyme and Xylanase. | ||
| + | |||
=Material and methods= | =Material and methods= | ||
| Line 94: | Line 89: | ||
=References= | =References= | ||
| + | [1] Watanabe, M., Yuzawa, H., Handa, N., & Kobayashi, I. (2006). Hyperthermophilic DNA methyltransferase M.PabI from the archaeon Pyrococcus abyssi. Applied and Environmental Microbiology, 72(8), 5367–75. | ||
| + | [2] Meissner, A. et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–70 (2008) | ||
Revision as of 13:44, 14 October 2014
Contents |
Introduction
Motivation
The invention of the polymerase chain reaction in 1983 by Kary Mulliy revolutionized the modern world by enabling amplification of DNA in an exponential manner. Further improvements including the use of thermo-stable DNA-polymerases made the method even more efficient, allowing its widespread use in nearly every field of modern diagnostics and research. However a major part of information is lost when using conventional PCR due to missing transfer of DNA modifications. Those covalently attached functional groups are present all kingdoms of life and act as prominent regulators of gene expression, adding an additional level of complexity.
We propose the PCR 2.0 as an easy and efficient way to amplify DNA templates in an exponential manner while maintaining their specific methylation pattern. The pivotal element of this PCR reaction is the use of a heat stable DNA methyltransferase (DNMT). Although comparable enzymes exist in thermophile organisms [1] until now no suitable protein could be found or synthesized that withstands the harsh conditions of a PCR.
Even though the detection and mapping of methylation patterns has become feasible in a high-throughput manner by using bisulfite sequencing and array techniques [2], further functional analysis is still limited due to the small amount of primary material. Our approach therefore aims to provide unlimited amounts of any methylated DNA sample of interest, withouth having to know its actual structure or the need of expensive methods and de-novo synthesis – just by running a PCR.
Hence, main goal of our project has been the generation of a heat stable Dnmt that can be produced in a large scale and shows efficient and specific methylation of DNA. Our approach applies protein circularization by using self-splicing protein sequences, socalled inteins from our toolbox as well as our linker design software (links). Since the restriction of conformational changes through intramolecular bonds has been known to increase the stability of proteins (Vieille, C. & Zeikus, G. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65, 1–43 (2001); Antos, J. M., Popp, M. W.-L., Ernst, R., Chew, G.-L., Spooner, E., & Ploegh, H. L. (2009). A straight path to circular proteins. The Journal of Biological Chemistry, 284(23), 16028–36.) and joining of C and N terminus were reported to increases in thermostability of smaller peptides (Tam, J. P. & Wong, C. T. T. Chemical synthesis of circular proteins. J. Biol. Chem. 287, 27020–5 (2012) references xylanase gfp…) we started the circularization of the so far biggest protein…
Epigenetics - there is more than just ATC and G…
In mammals and other vertebrates the methylation of cytosine nucleotides that are followed by guanines (CpG) is a prominent key regulator of transcription, embryonic development, X chromosome inactivation and many other cellular functions. (Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005); Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 Suppl, 245–54 (2003).). Approximately 1% of the genetic code in human somatic cells is consisting of methylated cytosines, amounting to 70-80% of all present CpG Dinucleotides. (Ehrlich, M. 1982. Amount and distribution of 5-methycytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 10: 2709–2721.) Due to this great prevalence and the heredity of this modification, the 5-methylcytosine (5mC) is also known as the “fifth base” of eukaryotic genomes. (Lister, R. & Ecker, J. Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome Res. 959–966 (2009)). DNA methylation is preferentially occurring at intergenic regions and repetitive sequences, where it is known to silence gene expression (Bird, A. (2002). DNA methylation patterns and epigenetic memory. Genes & Development 16, 6-21.); Jaenisch, R., and Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33, 245-254.) As against that, GC-rich promoter regions are usually characterized by a low methylation status, allowing transcription of the associated genes (Weichenhan, D., and Plass, C. (2013). The evolving epigenome. Human Molecular Genetics 22, R1. Disregulations of Cytosine methylation have been reported to play a crucial role in the development of numerous diseases, including cancer, imprinting diseases and repeat-instability diseases including morbus huntington (Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005))
A closer look at DNA Methyltransferases
The family of enzymes called DNA methyltransferases (DNMTs) is responsible for the establishment and maintenance of most cell-type specific DNA methylation patterns. Whereas the two enzymes DNMT3a and DNMT3b contribute to de novo methylation of DNA during development, DNMT1 is responsible to preserve existing methylation patterns throughout cell divisions. Therefore DNMT1 exploits the principle of semiconservative replication, using the parental strand as a template to create an exact copy of methylation patterns. Subsequent to recognition of a socalled hemi-methylated CpG site, the enzymatic machinery transferres a methyl group from the methyl donor S-Adeosyl-methionine (SAM) to the C5 position of the complementary cytosine residue. (Jurkowska, R. Z., Jurkowski, T. P. & Jeltsch, A. Structure and function of mammalian DNA methyltransferases. Chembiochem 12, 206–22 (2011).)
For our experiments we used the smallest truncated version of DNMT1 that has been reported to efficiently mediate methylation maintenance. This truncated version derives from Mus musculus and comprises amino acids 731-1602 (mDNMT1(731-1602) Song, J., Teplova, M., Ishibe-Murakami, S. & Patel, D. J. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science 335, 709–12 (2012).; Bashtrykov, P. et al. Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem. Biol. 19, 572–8 (2012)) kindly provided by Dr. Bashtrykov after approval). mDNMT1(731-1602) contains the C-terminal catalytic methyltransferasedomain as well as the aligning bromo-adjacent homology (BAH) domains, which prevent the binding of unmethylated DNA in the catalytic core. The enzyme is missing the CXXC domain, which has a high affinity to CpG hemi-methylated dinucleotides, but was shown to be dispensable for protein function (Bashtrykov, P., Jankevicius, G., Smarandache, A., Jurkowska, R. Z., Ragozin, S., & Jeltsch, A. (2012). Specificity of DNMT1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chemistry & Biology, 19(5), 572–8.). The truncated version has similar methylation activity and specificity as the wild type, as shown by our own experiments and by Bashtrykov and colleagues. In contrast to the full-length DNMT1 that can only be expressed in insect cells using a baculovirus based system, the mDNMT1(731-1602) has the great advantage of being efficiently expressed in E.coli. Since the N and C terminal ends of DNMT1 (731-1602) are closer together than in the full-lengh DNMT1, cirularisation might cause less deformation and have a lower impact on the overall activity, making this truncated mDNMT1 an ideal target for our purposes. (Figure: cristal structure)
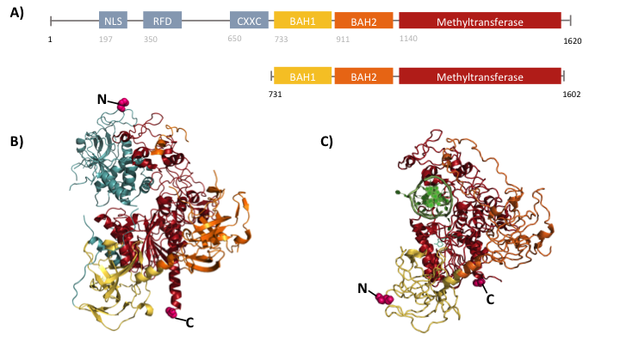
Structural overview of full length mDNMT1 and truncated mDNMT1(650–1602) (A) Color-coded domain devision and numbering of mDNMT1 sequence. (B) Ribbon presentation of full-length DNMT1 with the CXXC,BAH1, BAH2,and methyltransferase domain colored in light blue, yellow, orange and red, respectively. (C) Crystal structure of truncated DNMT1, missing the CXXC domain. Distant between terminal ends of the proteins are closer than at the full length version.
Circularization - The missing link
However, even the truncated DNMT1 is a very large protein of 100kDa and consists of 872 amino acids. To our knowledge, a protein this size has never been circularized before. According to the crystal structure, the distance between the termini of the truncated DNMT1 is 48 Å. We suspected that circularization of the protein by direct fusion of both termini might cause deformation of the protein structure. Therefore we generated a linker amino acid sequence, adapted to the structure of DNMT1: it needed to be long enough to bridge the gap of 48 Å and should bypass the catalytic core located prominently in the middle of the enzyme structure. Since we were not able to estimate the impact of the linker introduction, we focused on two different kinds of linkers: a rather rigid linker that has been calculated and optimized during the establishment of our linker and a flexible peptide connection consisting mostly of Glycine and Serine amino acids. To perform circularization we have used the split NpuDnaE Intein since it is known as the golden standard of protein splicing and has been efficiently tested by us through circularization of GFP, Lysosyme and Xylanase.
Material and methods
Results
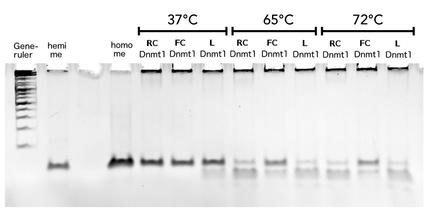
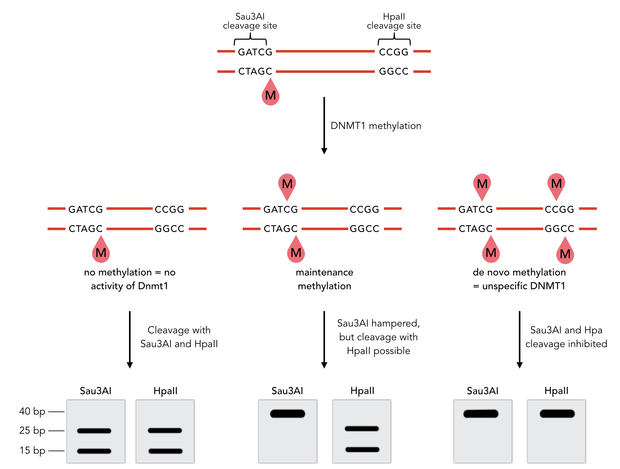
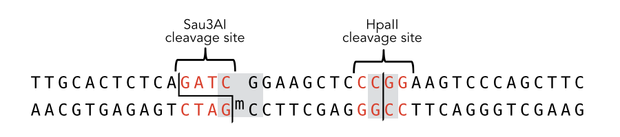
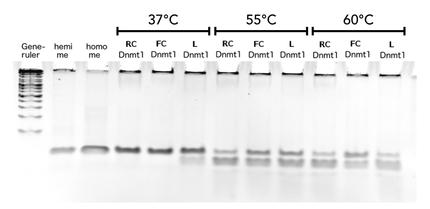
Circular DNMT1 with rigid linker (RC), circular DNMT1 with flexible linker (FC) and linear DNMT1 were heat shocked for 5 sec at 65°C and 72°C.Methylation after one hour was measured. A darker upper band for FC is even visible by eye, indication higher methylation activity.
References
[1] Watanabe, M., Yuzawa, H., Handa, N., & Kobayashi, I. (2006). Hyperthermophilic DNA methyltransferase M.PabI from the archaeon Pyrococcus abyssi. Applied and Environmental Microbiology, 72(8), 5367–75. [2] Meissner, A. et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–70 (2008)
 "
"