Team:Freiburg/Content/Results/Receptor
From 2014.igem.org
| Line 86: | Line 86: | ||
whether the tags have an impact on viral infection capabilities, we tested them for | whether the tags have an impact on viral infection capabilities, we tested them for | ||
their functionality, confirming that all receptor constructs lead to viral infection | their functionality, confirming that all receptor constructs lead to viral infection | ||
| - | when expressed in non-murine cell lines (Fig. | + | when expressed in non-murine cell lines (Fig. 3). For making the receptor visible for |
fluorescent microscopy and analysis by flow cytometry, we used the mCherry tag | fluorescent microscopy and analysis by flow cytometry, we used the mCherry tag | ||
(constructs p14rz_005 and p14rz_006). | (constructs p14rz_005 and p14rz_006). | ||
| Line 98: | Line 98: | ||
<figcaption> | <figcaption> | ||
<p class="header"> | <p class="header"> | ||
| - | Figure | + | Figure 3: HEK-293T cells transfected with different receptor constructs |
and infected with MuLV-EGFP afterwards. | and infected with MuLV-EGFP afterwards. | ||
</p> | </p> | ||
<p class="desc"> | <p class="desc"> | ||
| - | Cells transfected with (A) | + | Cells transfected with (A) mCAT-1, (B) mCAT-1-HA and (C) mCAT-1-HA-mCherry (red). |
Cells were transduced with MuLV-EGFP after 24 h. Pictures were taken after 48 h. | Cells were transduced with MuLV-EGFP after 24 h. Pictures were taken after 48 h. | ||
EGFP fluorescence is shown in green. | EGFP fluorescence is shown in green. | ||
| Line 113: | Line 113: | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<p> | <p> | ||
| - | + | Owing to the limited resolution and sensitivity of the cell culture microscope used | |
| - | + | for the images in Fig. 3, it was not clear whether the cells expressing the receptor | |
| - | the | + | were the same cells that were transduced by the virus and expressed EGFP (Fig. 3C). |
| - | + | Therefore we obtained higher qualtiy images with a confocal microscope (Fig. 4). The | |
| + | cells were transfected with the mCAT-1-mCherry construct, 24h later infected by | ||
| + | the virus containing EGFP, and imaged 48h later. We found many cells that expressed | ||
| + | EGFP in the cytoplasm and the mCAT-1 receptor on the plasma membrane, whereas we did not | ||
| + | find cells that expressed EGFP but not the receptor, suggesting that | ||
| + | mCAT-1 expression is required for infection of the cells by the MuLV. | ||
</p> | </p> | ||
| + | <p> | ||
| + | Regarding the expression rate of the receptor on the surface of HEK293T cells, | ||
| + | we noticed that mCAT-1-mCherry is less present after transduction with | ||
| + | our viral particles. That means that the receptor is eather internalized | ||
| + | after infection by a mechanism initiated by the virus, or the expression | ||
| + | rate of the receptor decreases after infection. Since the particles can only | ||
| + | transfer their cargo into dividing cells we found often pairs of cells that were | ||
| + | infected by the viral vector or at least high expression rate of the receptor in | ||
| + | cells next to targeted cells. | ||
| + | </p> | ||
| + | |||
</div> | </div> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
| Line 127: | Line 143: | ||
<figcaption> | <figcaption> | ||
<p class="header"> | <p class="header"> | ||
| - | Figure | + | Figure 4: HEK293T cells expressing mCAT-1-mCherry transduced with MuLV-GFP. |
</p> | </p> | ||
<p class="desc"> | <p class="desc"> | ||
| - | Overlay of all three channels ( | + | (A) Overlay of all three channels, (B) DAPI, (C) EGFP and (D) mCAT-1-mCherry. |
| - | + | Objective plan apo 60x, NA 1.40. | |
| - | + | ||
</p> | </p> | ||
</figcaption> | </figcaption> | ||
| Line 171: | Line 186: | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 19:43, 17 October 2014
The Receptor
The specificity of our system is based on the murine CAT-1 receptor, which serves as the viral entry site of our viral vector into the target cells. We demonstrate that the virus can be used to stably integrate genes into the genome of murine cells. We also targeted other cell lines by expressing the gene for the murine CAT-1 receptor in these cells before viral transduction. Since mCAT-1 is naturally only present in murine cell lines, we use human cell lines for transfection of the receptor. Expression of a reporter suggests that only the cells expressing the mCAT-1 receptor are being infected by the viral particles, thus making particular gene transfer possible.
Subcellular Localization of the Receptor
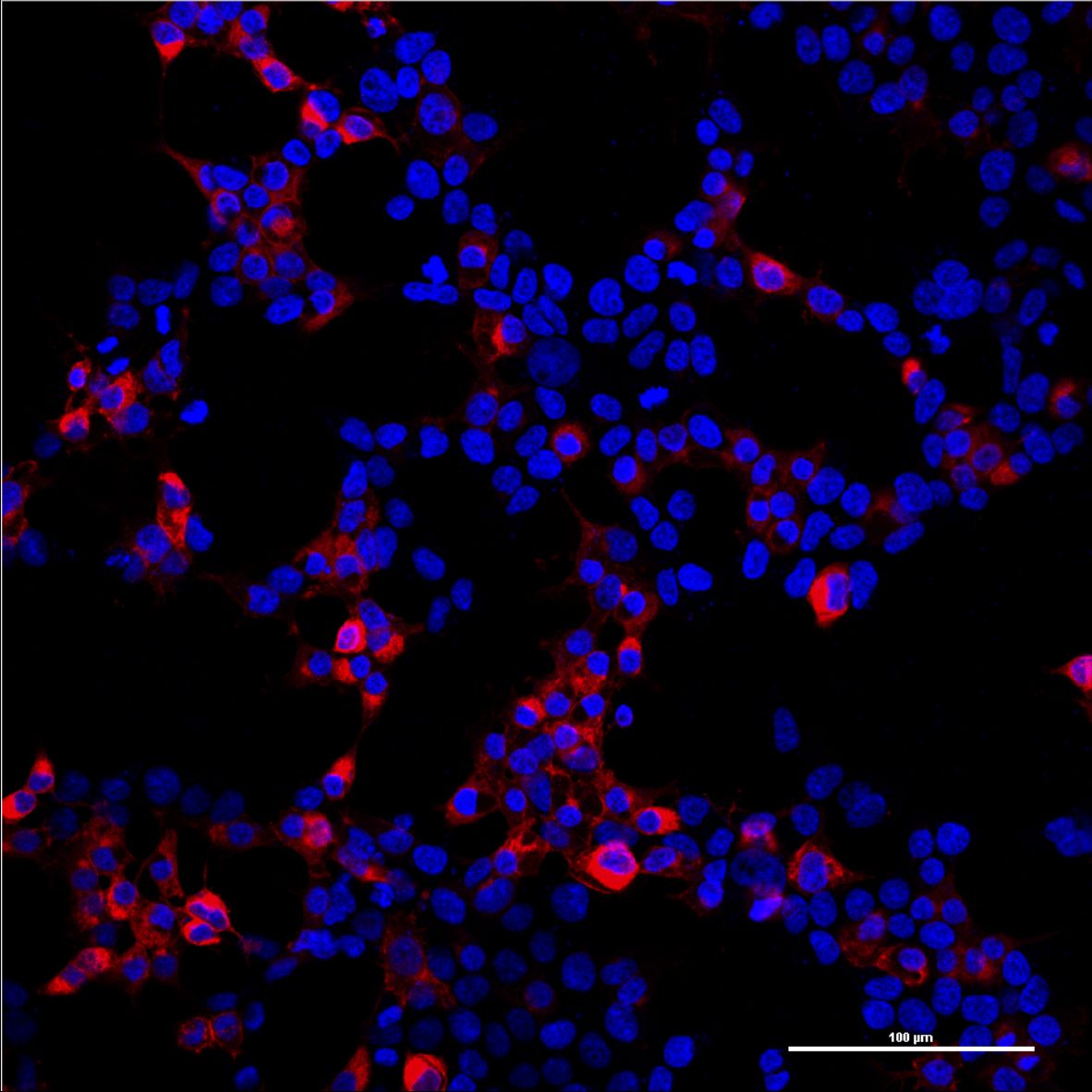
Since the mCAT-1 receptor serves as the entry site of our viral vector, it is essential that it is expressed on the surface of target cells. In order to determine the localization of the mCAT-1 receptor, we labeled the C-terminus of the mCAT-1 with the fluorescent protein mCherry and transfected it into human embryonic kidney (HEK-293T) cells. After distinct time points cells were imaged with a confocal scanning laser microscope. The mCAT1-mCherry construct was found predominantly at the surface of the cells (Figs. 1, 2).
Receptor Functionality
Transduction of genes into murine cell lines that naturally express the mCAT-1 receptor occurred with an efficiency of over 80%. For our system we needed functional expression of the receptor in non-murine cell lines, i.e. the receptor has to serve as an entry site for the virus, and the virus has to efficiently deliver its cargo into the target cell. We tested the functionality of the mCAT-1 receptor by expressing it in HEK-293T cells and subsequent infection with the virus containing EGFP as a cargo. The presence of green fluorescent cells in the infected cultures of different non-murine cell lines indicated that the receptor was not only expressed but can also be used as an entry site by the virus.
During our project we generated receptor constructs which were labeled with fluorescent protein (for microscopy) or the HA tag (for Western Blotting). Since we didn't know whether the tags have an impact on viral infection capabilities, we tested them for their functionality, confirming that all receptor constructs lead to viral infection when expressed in non-murine cell lines (Fig. 3). For making the receptor visible for fluorescent microscopy and analysis by flow cytometry, we used the mCherry tag (constructs p14rz_005 and p14rz_006).

Figure 3: HEK-293T cells transfected with different receptor constructs and infected with MuLV-EGFP afterwards.
Cells transfected with (A) mCAT-1, (B) mCAT-1-HA and (C) mCAT-1-HA-mCherry (red). Cells were transduced with MuLV-EGFP after 24 h. Pictures were taken after 48 h. EGFP fluorescence is shown in green.
Owing to the limited resolution and sensitivity of the cell culture microscope used for the images in Fig. 3, it was not clear whether the cells expressing the receptor were the same cells that were transduced by the virus and expressed EGFP (Fig. 3C). Therefore we obtained higher qualtiy images with a confocal microscope (Fig. 4). The cells were transfected with the mCAT-1-mCherry construct, 24h later infected by the virus containing EGFP, and imaged 48h later. We found many cells that expressed EGFP in the cytoplasm and the mCAT-1 receptor on the plasma membrane, whereas we did not find cells that expressed EGFP but not the receptor, suggesting that mCAT-1 expression is required for infection of the cells by the MuLV.
Regarding the expression rate of the receptor on the surface of HEK293T cells, we noticed that mCAT-1-mCherry is less present after transduction with our viral particles. That means that the receptor is eather internalized after infection by a mechanism initiated by the virus, or the expression rate of the receptor decreases after infection. Since the particles can only transfer their cargo into dividing cells we found often pairs of cells that were infected by the viral vector or at least high expression rate of the receptor in cells next to targeted cells.
Analysis of cells expressing mCAT-1-mCherry that were infected with MuLV EGFP showed that all cells which were transduced by the viral particles also express the receptor, i.e. only cells expressing the receptor were infected (Fig. 3).
Receptor Expression
The time point for viral infection was adjusted to the time the receptor needs for expression in target cells. In order to determine the expression time of mCAT-1 in HEK-293T cells, we transfected the cells with the HA-tagged mCAT-1 (p14rz_006). Cells transfected with receptor DNA were analyzed after different incubation times. As the expression of the receptor peaks at 24 h after transfection, we used this time point for viral infections (Fig. 6). Since the presence of mCAT-1 on the cell surface is a main factor for viral tranduction efficiency we tested the expression rate of the receptor after transfection of HEK293T cells with different receptor DNA concentrations with Western blot. Although we determined that the presence of the receptor in the cell increases, if more DNA was transfected into the cells, we did not uses such high DNA concentrations. We found that non-murine cells transfected with high amoounts of receptor DNA die due to overexpression of mCAT-1.

Figure 6: Expression time of the receptor that was transfected into HEK293 cells.
After transfection with p14rz_006 (HA-labeled) cells were lysed with RIPA buffer at distinct time points. A Western blot was performed using anti-HA antibody.

Figure 7: Tranfection of HEK-293T cells with different receptor DNA concentrations.
Cells (on 35mm plates) were transfected with 0,6 µg to 5,4 µg p14rz_006 (HA-labeled) per well. Cells were lysed with RIPA buffer after 24 h of incubation and analysed by Western blotting.
 "
"