Team:Freiburg/Content/Results/Receptor
From 2014.igem.org
Mirja Harms (Talk | contribs) |
NatalieLouis (Talk | contribs) |
||
| Line 9: | Line 9: | ||
<h1>The Receptor</h1> | <h1>The Receptor</h1> | ||
| - | <p>The specificity of our system is based on the murine CAT-1 receptor which serves as the viral entry site of our vector into target cells. We could show that our viral vector system can be used to stably integrate genes into the genome of murine cells. We could also target any other cell line by transferring the gene for the murine CAT-1 receptor in these cells before transduction. Since mCAT-1 is naturally only present in murine cell lines, we use human cell lines for transfection of the receptor gene. Expression of a reporter demonstrates that only the cells expressing the mCAT-1 receptor are infected by the viral particles, thus making particular gene transfer possible. | + | <p>The specificity of our system is based on the murine CAT-1 receptor, which serves as the viral entry site of our vector into target cells. We could show that our viral vector system can be used to stably integrate genes into the genome of murine cells. We could also target any other cell line by transferring the gene for the murine CAT-1 receptor in these cells before transduction. Since mCAT-1 is naturally only present in murine cell lines, we use human cell lines for transfection of the receptor gene. Expression of a reporter demonstrates that only the cells expressing the mCAT-1 receptor are infected by the viral particles, thus making particular gene transfer possible. |
</p> | </p> | ||
| Line 16: | Line 16: | ||
<h2 id="Results-Receptor-LocalisationReceptor">Localisation Receptor</h2> | <h2 id="Results-Receptor-LocalisationReceptor">Localisation Receptor</h2> | ||
| - | <p>Since | + | <p>Since the mCAT-1 receptor serves as the entry site of our viral vector, it is essential that it is expressed on the surface of target cells. In order to determinate the localization of the mCAT-1 receptor, we labeled the C-terminus of the mCAT-1 with the fluorescence protein mCherry and transfected this construct in human embryonic kidney (HEK-293T) cells. After distinct time points cells were imaged with a confocal scanning laser microscope. The mCAT1-mCherry was found predominantly at the surface of the cells. |
</p> | </p> | ||
<h2 id="Results-Receptor-ReceptorFunctionality">Receptor Functionality</h2> | <h2 id="Results-Receptor-ReceptorFunctionality">Receptor Functionality</h2> | ||
| - | <p>Transduction of genes into murine cell lines which naturally express the mCAT-1 receptor occurred with an efficiency over 80%. For our system we need a functional expression of the receptor in non-murine cell lines, i.e. the receptor has to serve as an entry site for the virus, and the virus has to efficiently deliver its cargo into the target cell. We tested the functionality of the mCAT-1 receptor by infecting | + | <p>Transduction of genes into murine cell lines which naturally express the mCAT-1 receptor occurred with an efficiency over 80%. For our system we need a functional expression of the receptor in non-murine cell lines, i.e. the receptor has to serve as an entry site for the virus, and the virus has to efficiently deliver its cargo into the target cell. We tested the functionality of the mCAT-1 receptor by infecting HEK-293T cells expressing the receptor with the virus containing eGFP as a cargo. The presence of green fluorescent cells in the infected cultures of different non-murine cell lines indicates that the receptor is not only expressed but can be also used as an entry site by the virus. |
</p> | </p> | ||
| - | <p>During our projekt we generated different receptor constructs all of them labeled with differnt markers. We tested all of them for their functionality (Fig.). Analysis with flow cytometry | + | <p>During our projekt we generated different receptor constructs all of them labeled with differnt markers. We tested all of them for their functionality (Fig.). Analysis with flow cytometry showed that all cells which were transduced by the viral particles transferring eGFP also express mCherry, i.e. only cells expressing the receptor were infected. |
</p> | </p> | ||
| Line 31: | Line 31: | ||
</a> | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header">Figure : | + | <p class="header">Figure : HEK-293T cells transfected with different receptor constructs and infected with MuLV eGFP afterwards.</p> |
| - | <p class="desc">The cells were transfected with pQCXIN (original vector containing SLC7A1) (A), p14rz_004 (labeled with HA-tag)(B) and p14rz_006 (labeled with HA-tag and mCherry)(C). Cells were transduced with MuLV after 24 | + | <p class="desc">The cells were transfected with pQCXIN (original vector containing SLC7A1) (A), p14rz_004 (labeled with HA-tag)(B) and p14rz_006 (labeled with HA-tag and mCherry)(C). Cells were transduced with MuLV after 24 h. Pictures were taken after 48 h.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 49: | Line 49: | ||
</a> | </a> | ||
<figcaption> | <figcaption> | ||
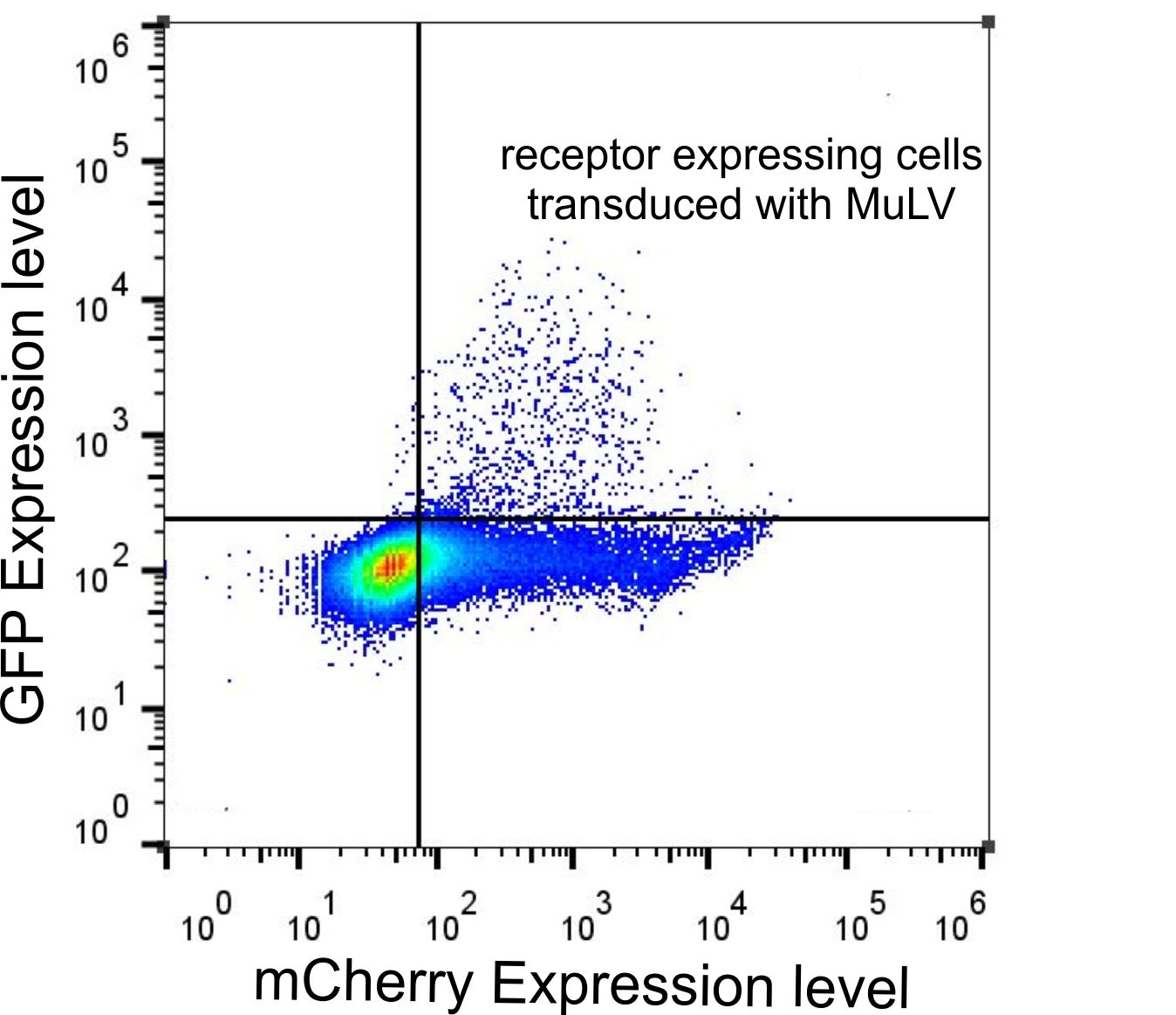
| - | <p class="header">FACS data of | + | <p class="header">FACS data of HEK-293T cells tranfected with the receptor and transduced with MuLV eGFP.</p> |
| - | <p class="desc">HEK cells were transfected with the p14rz_006 | + | <p class="desc">HEK-293T cells were transfected with the p14rz_006 which was labeled with mCherry and infected with MuLV transferring eGFP. Cells were analyzed with flow cytometry 48 h after transduction.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 58: | Line 58: | ||
<h2 id="Results-Receptor-ReceptorExpression">Receptor Expression</h2> | <h2 id="Results-Receptor-ReceptorExpression">Receptor Expression</h2> | ||
| - | <p>The time point for viral infection was adjusted to the time the receptor needs for expression in target cells. In order to determine the expression time of mCAT-1 in | + | <p>The time point for viral infection was adjusted to the time the receptor needs for expression in target cells. In order to determine the expression time of mCAT-1 in HEK-293T cells, we transfected the cells with the HA-tagged mCAT-1 (p14rz_006). Cells transfected with receptor DNA were analyzed after different incubation times. As the expression of the receptor peaks at 24 h after transfection, we used this time point for viral infections. |
</p> | </p> | ||
| Line 67: | Line 67: | ||
<figcaption> | <figcaption> | ||
<p class="header">Expression time of the receptor that was transfected into HEK293 cells.</p> | <p class="header">Expression time of the receptor that was transfected into HEK293 cells.</p> | ||
| - | <p class="desc">After transfection with p14rz_006 (HA-labeled) cells were lysed with | + | <p class="desc">After transfection with p14rz_006 (HA-labeled) cells were lysed with RIPA buffer at distinct time points. A Western blot was performed using anti-HA antibody.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 76: | Line 76: | ||
<img src="https://static.igem.org/mediawiki/2014/c/cc/Freiburg2014_Results_WB_receptorexpression_DNA_conc.jpg"> | <img src="https://static.igem.org/mediawiki/2014/c/cc/Freiburg2014_Results_WB_receptorexpression_DNA_conc.jpg"> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header">Tranfection of | + | <p class="header">Tranfection of HEK-293T cells with different receptor DNA concentrations.</p> |
| - | <p class="desc">Cells (on 35mm plates) were transfected with 0,6 µg to 5,4 µg p14rz_006 (HA-labeled) per well. Cells were lysed with RIPA buffer after 24 | + | <p class="desc">Cells (on 35mm plates) were transfected with 0,6 µg to 5,4 µg p14rz_006 (HA-labeled) per well. Cells were lysed with RIPA buffer after 24 h of incubation and analysed by Western blotting.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
Revision as of 08:56, 17 October 2014
The Receptor
The specificity of our system is based on the murine CAT-1 receptor, which serves as the viral entry site of our vector into target cells. We could show that our viral vector system can be used to stably integrate genes into the genome of murine cells. We could also target any other cell line by transferring the gene for the murine CAT-1 receptor in these cells before transduction. Since mCAT-1 is naturally only present in murine cell lines, we use human cell lines for transfection of the receptor gene. Expression of a reporter demonstrates that only the cells expressing the mCAT-1 receptor are infected by the viral particles, thus making particular gene transfer possible.
Localisation Receptor
Since the mCAT-1 receptor serves as the entry site of our viral vector, it is essential that it is expressed on the surface of target cells. In order to determinate the localization of the mCAT-1 receptor, we labeled the C-terminus of the mCAT-1 with the fluorescence protein mCherry and transfected this construct in human embryonic kidney (HEK-293T) cells. After distinct time points cells were imaged with a confocal scanning laser microscope. The mCAT1-mCherry was found predominantly at the surface of the cells.
Receptor Functionality
Transduction of genes into murine cell lines which naturally express the mCAT-1 receptor occurred with an efficiency over 80%. For our system we need a functional expression of the receptor in non-murine cell lines, i.e. the receptor has to serve as an entry site for the virus, and the virus has to efficiently deliver its cargo into the target cell. We tested the functionality of the mCAT-1 receptor by infecting HEK-293T cells expressing the receptor with the virus containing eGFP as a cargo. The presence of green fluorescent cells in the infected cultures of different non-murine cell lines indicates that the receptor is not only expressed but can be also used as an entry site by the virus.
During our projekt we generated different receptor constructs all of them labeled with differnt markers. We tested all of them for their functionality (Fig.). Analysis with flow cytometry showed that all cells which were transduced by the viral particles transferring eGFP also express mCherry, i.e. only cells expressing the receptor were infected.

Figure : HEK-293T cells transfected with different receptor constructs and infected with MuLV eGFP afterwards.
The cells were transfected with pQCXIN (original vector containing SLC7A1) (A), p14rz_004 (labeled with HA-tag)(B) and p14rz_006 (labeled with HA-tag and mCherry)(C). Cells were transduced with MuLV after 24 h. Pictures were taken after 48 h.
Receptor Expression
The time point for viral infection was adjusted to the time the receptor needs for expression in target cells. In order to determine the expression time of mCAT-1 in HEK-293T cells, we transfected the cells with the HA-tagged mCAT-1 (p14rz_006). Cells transfected with receptor DNA were analyzed after different incubation times. As the expression of the receptor peaks at 24 h after transfection, we used this time point for viral infections.

Expression time of the receptor that was transfected into HEK293 cells.
After transfection with p14rz_006 (HA-labeled) cells were lysed with RIPA buffer at distinct time points. A Western blot was performed using anti-HA antibody.

Tranfection of HEK-293T cells with different receptor DNA concentrations.
Cells (on 35mm plates) were transfected with 0,6 µg to 5,4 µg p14rz_006 (HA-labeled) per well. Cells were lysed with RIPA buffer after 24 h of incubation and analysed by Western blotting.
 "
"