Team:Austin Texas/kit
From 2014.igem.org
| Line 206: | Line 206: | ||
[[File:UT_Austin_2014_Kit_Incorporation_Value_Graph.png|600px|thumb|'''Figure 5.''' Incorporation values for each synthetase/tRNA pair. The dashed line equals an incorporation value of 1. Values greater than 1 when using pFRY are desirable. The GFP:RFP relative fluorescence values in the presence and absence of ncAA (see '''Figure 4''') were used to calculate incorporation values. Values were determined by dividing the GFP:RFP relative fluorescence in the presence of ncAA by the GFP:RFP relative fluorescence in the absence of ncAA. This value does not correct for ncAA-related effects on translation, which can occasionally be seen with the pFRYC controls.]] | [[File:UT_Austin_2014_Kit_Incorporation_Value_Graph.png|600px|thumb|'''Figure 5.''' Incorporation values for each synthetase/tRNA pair. The dashed line equals an incorporation value of 1. Values greater than 1 when using pFRY are desirable. The GFP:RFP relative fluorescence values in the presence and absence of ncAA (see '''Figure 4''') were used to calculate incorporation values. Values were determined by dividing the GFP:RFP relative fluorescence in the presence of ncAA by the GFP:RFP relative fluorescence in the absence of ncAA. This value does not correct for ncAA-related effects on translation, which can occasionally be seen with the pFRYC controls.]] | ||
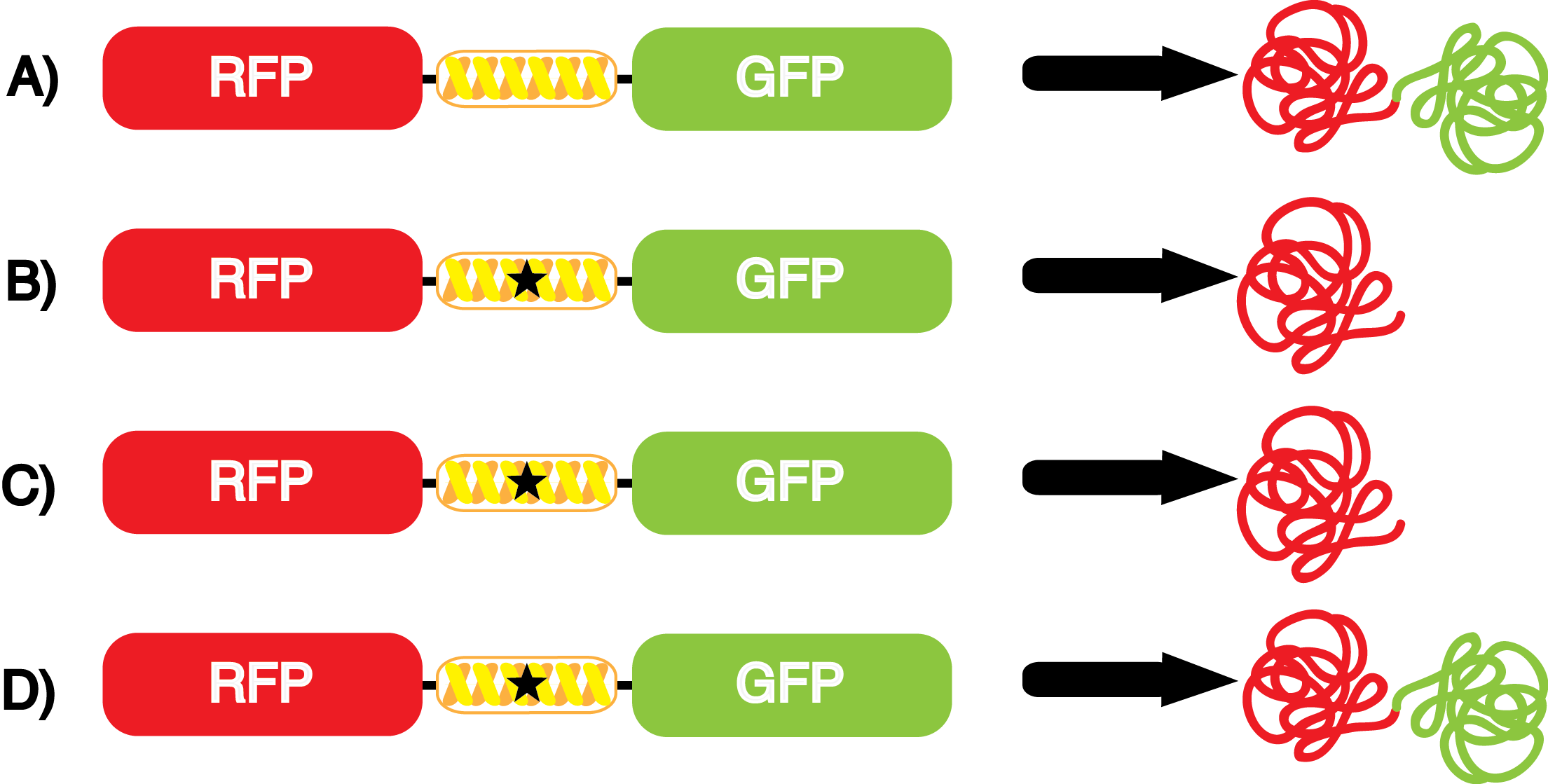
| - | + | We wanted a way to better summarize the output of the kit with a single measurement value. We decided to calculate an overall '''incorporation value''' as the GFP:RFP relative fluorescence value in the presence of the ncAA divided by the GFP:RFP relative fluorescence value when the ncAA is not included in the culture. Thus, the incorporation value is a single number that measures the relative efficiency and fidelity of a ncAA tRNA synthetase/tRNA pair. A value of 1 indicates no significant dependence of incorporation on the presence of ncAA in the culture. The higher the value, the higher the fidelity and/or efficiency of the synthetase/tRNA pair. It is important to note that ncAA-related effects on growth and translation can also reduce the apparent incorporation value of a synthetase/tRNA pair below 1. | |
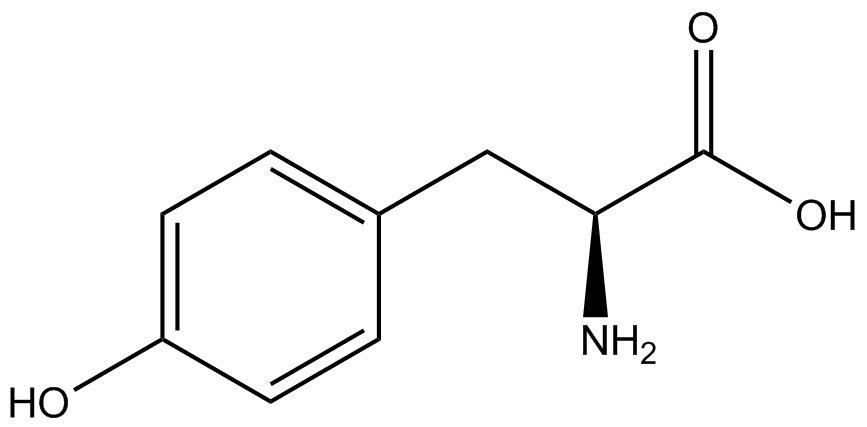
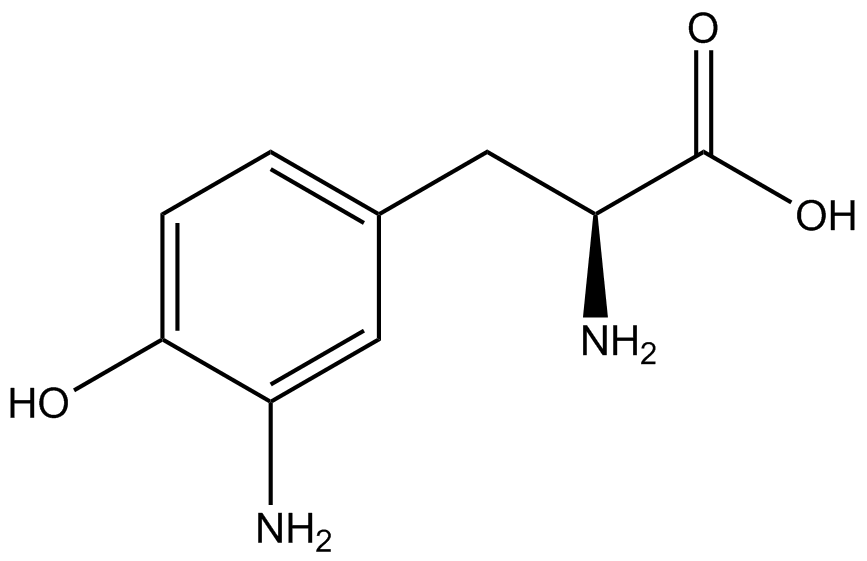
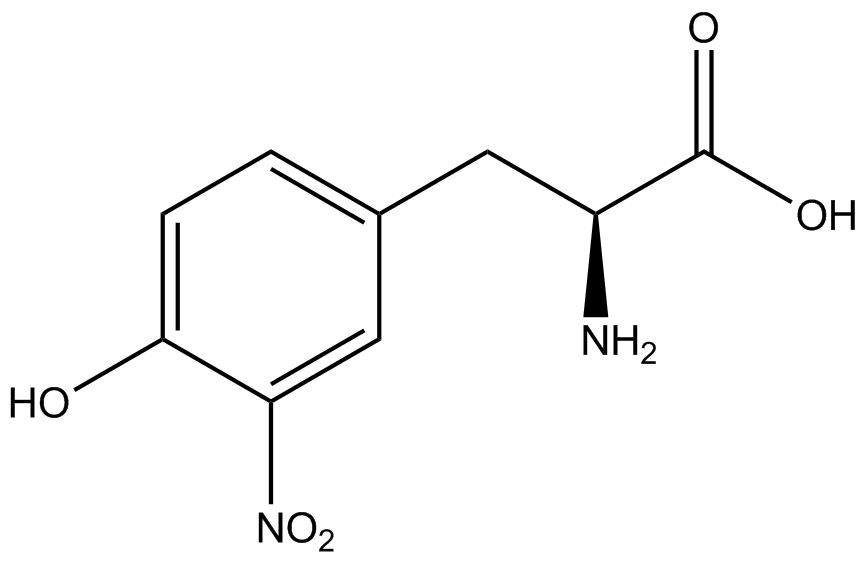
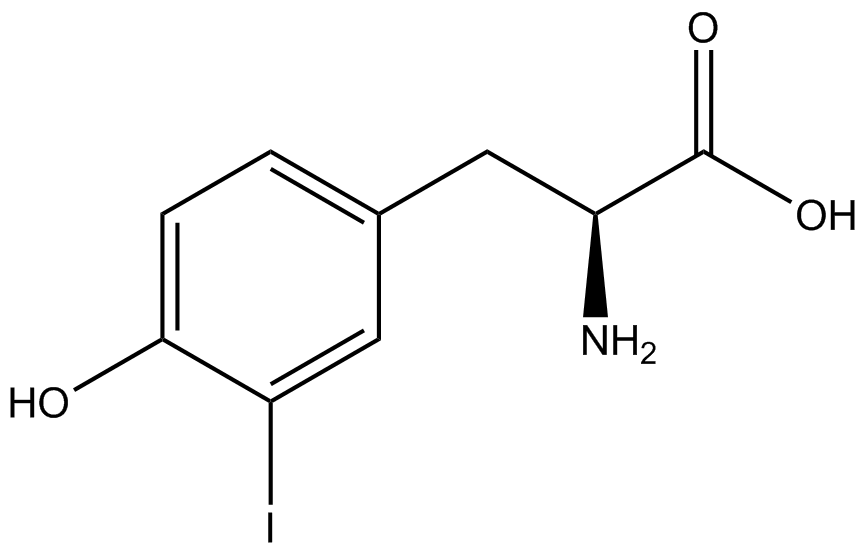
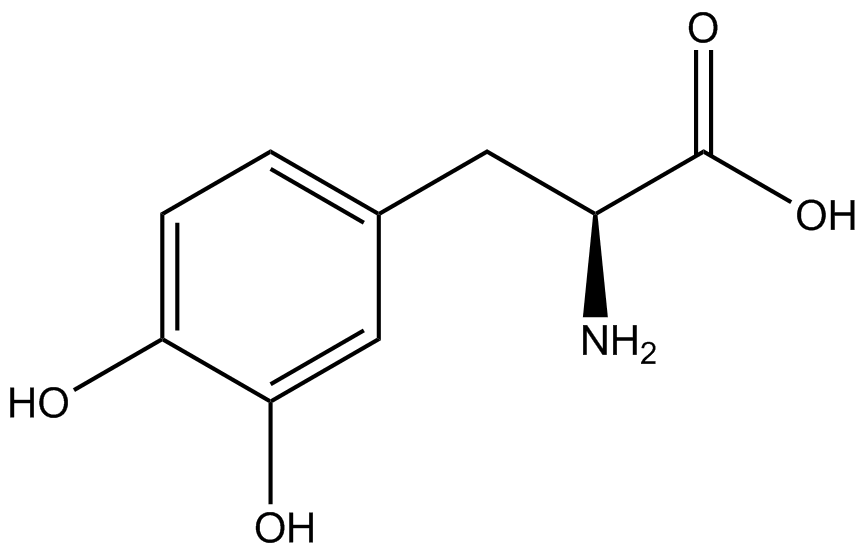
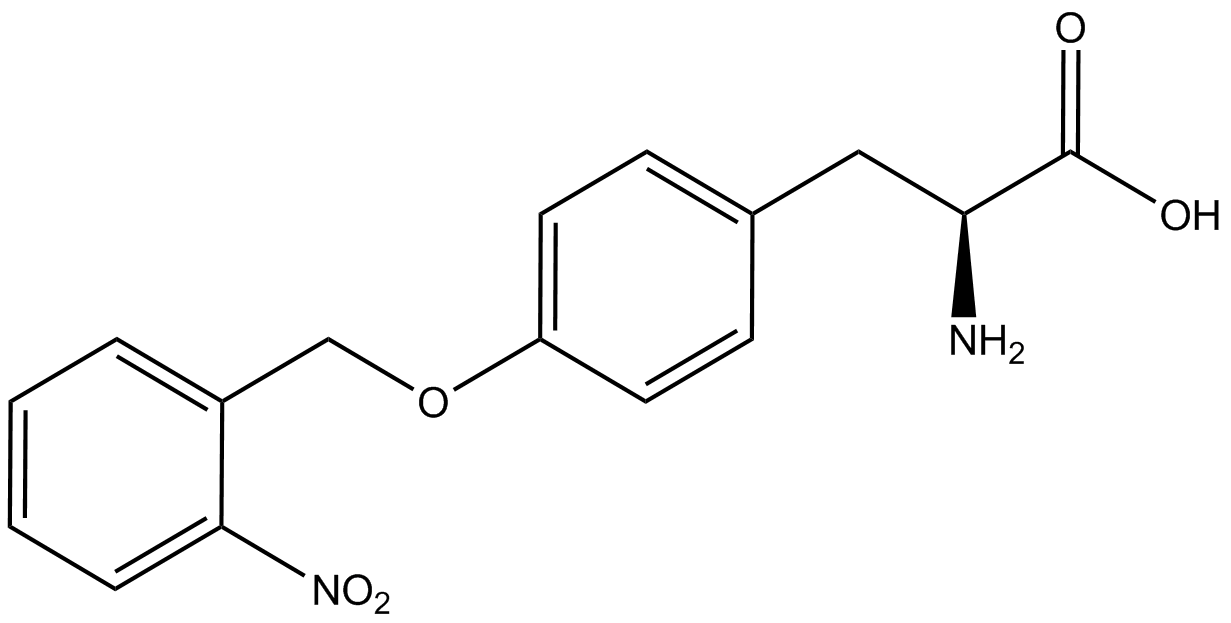
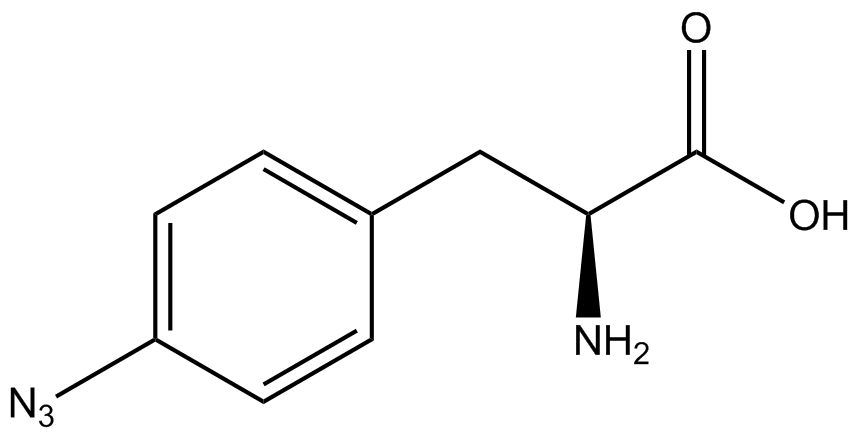
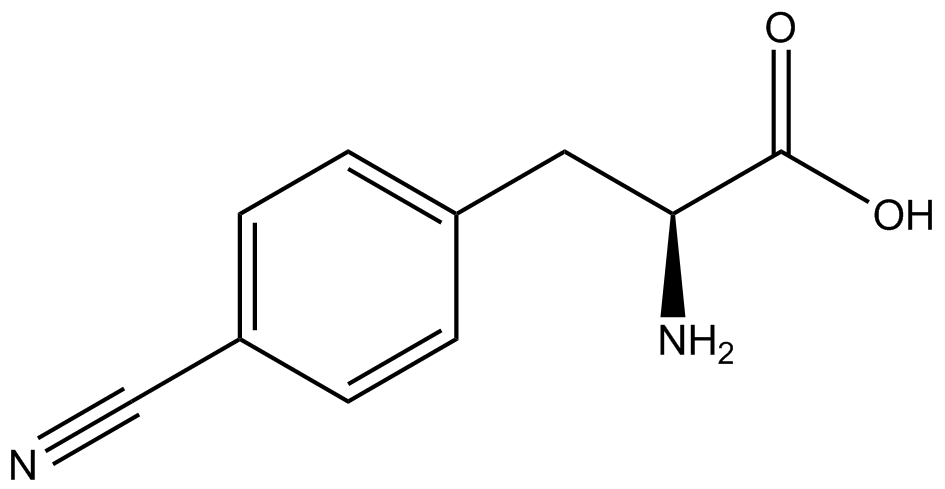
| - | The synthetase/tRNA pairs that showed the highest incorporation values include AzF, 3-nitrotyrosine, ONBY, and 3-iodotyrosine in decreasing order. Among the ncAAs tested, ONBY consistently slowed the growth rate of the culture significantly (as seen by | + | The synthetase/tRNA pairs that showed the highest incorporation values include AzF, 3-nitrotyrosine, ONBY, and 3-iodotyrosine in decreasing order. Among the ncAAs tested, ONBY consistently slowed the growth rate of the culture significantly (as seen by OD<sub>600</sub> readings, data not shown) suggesting possible toxicity to the cell. However, ncAA incorporation remained relatively high, resulting in a good incorporation value. Synthetase/tRNA pairs that showed the lowest levels of ncAA incorporation include 3-aminotyrosine, <small>L</small>-DOPA, and CNF. |
| Line 216: | Line 216: | ||
<h1>Discussion</h1> | <h1>Discussion</h1> | ||
| - | Our ncAA measurement kit was able to successfully compare the fidelity and efficiency of seven different synthetase/tRNA pairs. We were able to confidently say that one synthetase/tRNA pair had a higher fidelity than another. We saw clear evidence that three of our tRNA synthetase/tRNA pairs seemed incapable of discriminating between their ncAA and the 20 canonical amino acids present in media: 3-aminotyrosine, <small>L</small>-DOPA, and CNF. We were able to test triplicate cultures of seven different ncAAs and tyrosine (eight total synthetase/tRNA pairs) in one day, generating results that immediately told us which ncAAs worked best in our system both in terms of fidelity and efficiency of incorporation. This required no advanced training or access to equipment besides a shaker and a fluorometer. | + | Our ncAA measurement kit was able to successfully compare the fidelity and efficiency of seven different synthetase/tRNA pairs. We were able to confidently say that one synthetase/tRNA pair had a higher fidelity than another. '''We saw clear evidence that three of our tRNA synthetase/tRNA pairs seemed incapable of discriminating between their ncAA and the 20 canonical amino acids present in media: 3-aminotyrosine, <small>L</small>-DOPA, and CNF.''' We were able to test triplicate cultures of seven different ncAAs and tyrosine (eight total synthetase/tRNA pairs) in one day, generating results that immediately told us which ncAAs worked best in our system both in terms of fidelity and efficiency of incorporation. This required no advanced training or access to equipment besides a shaker and a fluorometer. |
| - | While the measurements may not be perfectly accurate due to the nature of this test, the measurement kit is designed more around ease of use, cost-efficiency, and portability. The ncAA measurement kit can be used to quickly test a synthetase/tRNA pair's quality with minimal effort, as opposed to other more accurate but more intensive or expensive measures such as mass spectrometry. While mass spectrometry can give much more definitive information about a synthetase/tRNA pair, there are many different factors that could make it unreasonable for undergraduate research. It is a technique that requires a fair amount of skill to do properly, and can be very costly as well. Additionally, the sample needs to be purified from cell culture and prepped for mass spectrometry. | + | While the measurements may not be perfectly accurate due to the nature of this test, the measurement kit is designed more around ease of use, cost-efficiency, and portability. The ncAA measurement kit can be used to quickly test a synthetase/tRNA pair's quality with minimal effort, as opposed to other more accurate but more intensive or expensive measures such as mass spectrometry. While mass spectrometry can give much more definitive information about a synthetase/tRNA pair, there are many different factors that could make it unreasonable for undergraduate research or high-throughput use. It is a technique that requires a fair amount of skill to do properly, and can be very costly as well. Additionally, the sample needs to be purified from cell culture and prepped for mass spectrometry. |
For the ncAA measurement kit, all that is necessary is to request from us the kit plasmids (pFRYC and PFRY in separate strains of amberless ''E. coli''), which we are immediately making available to anyone who requests the Expanded Genetic Code Measurement Kit. Once you receive the frozen cultures (or plasmids), transform the synthetase/tRNA pair that you wish to test into the two different cell strains, and run the experiment. Thus, if you receive the measurement kit on Monday, you could have your synthetase/tRNA pairs characterized by Friday. The kit can also be used to test how one or more synthetase/tRNA pairs work in different cells or under different conditions. However, in this case, you may need to retransform the kit plasmids into the different strain. | For the ncAA measurement kit, all that is necessary is to request from us the kit plasmids (pFRYC and PFRY in separate strains of amberless ''E. coli''), which we are immediately making available to anyone who requests the Expanded Genetic Code Measurement Kit. Once you receive the frozen cultures (or plasmids), transform the synthetase/tRNA pair that you wish to test into the two different cell strains, and run the experiment. Thus, if you receive the measurement kit on Monday, you could have your synthetase/tRNA pairs characterized by Friday. The kit can also be used to test how one or more synthetase/tRNA pairs work in different cells or under different conditions. However, in this case, you may need to retransform the kit plasmids into the different strain. | ||
| - | |||
| - | Due to the nature of the Expanded Genetic Code Measurement Kit, <i>in vivo</i> influences will affect certain data and results. This knowledge is very useful by providing a broader and biological | + | We are optimistic that this measurement kit can help speed up the process of developing a new synthetase/tRNA pairs, as well as characterizing large groups of synthetase/tRNA pairs, as we now have a way to quickly, cheaply, and easily do large scale characterizations. For example, we could now systematically test how changing the expression levels of a synthetase and its tRNA alters the efficiency and fidelity of ncAA incorporation. |
| + | |||
| + | Due to the nature of the Expanded Genetic Code Measurement Kit, <i>in vivo</i> influences will affect certain data and results. This knowledge is very useful by providing a broader and biological perspective of cellular health. In general, the presence of ncAAs slowed the growth of amberless ''E. coli'', sometimes quite significantly. This knowledge, as seen through the GFP:RFP ratio, can be very informative when assessing whether to use a ncAA tRNA synthetase/tRNA pair. | ||
<h1>Conclusion</h1> | <h1>Conclusion</h1> | ||
| - | This year the UT Austin iGEM Team has chosen to take part in the [https://2014.igem.org/Tracks/Measurement Measurement Track] of the iGEM competition. We have presented here the Expanded Genetic Code Measurement Kit for the purpose of quickly characterizing novel ncAA tRNA synthetase/tRNA pairs, which meet the [https://2014.igem.org/Team:Austin_Texas/medal_requirements medal criteria]. Prior to this measurement kit there was not a widely available and easily accessible kit for assessing ncAA tRNA synthetase/tRNA pairs. Although numerous labs have used mass spectrometry, such a technique is not always cheap not widely available. Additionally, we have begun the process of standardization for ncAA synthetase/tRNA pairs by using our kit to simultaneously characterize seven different ncAA synthetase/tRNA pairs as well as a tyrosine RS. '''This method of testing was designed to be easy-to-use, portable, quicker, and more available than other methods of characterization and analysis. We are optimistic that this is a first step in opening the doorway to | + | This year the UT Austin iGEM Team has chosen to take part in the [https://2014.igem.org/Tracks/Measurement Measurement Track] of the iGEM competition. We have presented here the Expanded Genetic Code Measurement Kit for the purpose of quickly characterizing novel ncAA tRNA synthetase/tRNA pairs, which meet the [https://2014.igem.org/Team:Austin_Texas/medal_requirements medal criteria]. Prior to this measurement kit there was not a widely available and easily accessible kit for assessing ncAA tRNA synthetase/tRNA pairs. Although numerous labs have used mass spectrometry, such a technique is not always cheap not widely available. Additionally, we have begun the process of standardization for ncAA synthetase/tRNA pairs by using our kit to simultaneously characterize seven different ncAA synthetase/tRNA pairs as well as a tyrosine RS. '''This method of testing was designed to be easy-to-use, portable, quicker, and more available than other methods of characterization and analysis. We are optimistic that this is a first step in opening the doorway to more iGEM teams and labs using these new chemical building blocks in their projects.''' |
Documentation, including sequence information, of the tRNA synthetase/tRNA pairs used can be found [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Synthetase_Table below]. | Documentation, including sequence information, of the tRNA synthetase/tRNA pairs used can be found [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Synthetase_Table below]. | ||
Revision as of 02:42, 18 October 2014
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"