Team:Austin Texas/kit
From 2014.igem.org
| Line 147: | Line 147: | ||
[[File:Alex and Foil-covered incubator.jpg|200px|thumb|right|Protection of light-sensitive ncAAs using a foil-wrapped incubator.]] | [[File:Alex and Foil-covered incubator.jpg|200px|thumb|right|Protection of light-sensitive ncAAs using a foil-wrapped incubator.]] | ||
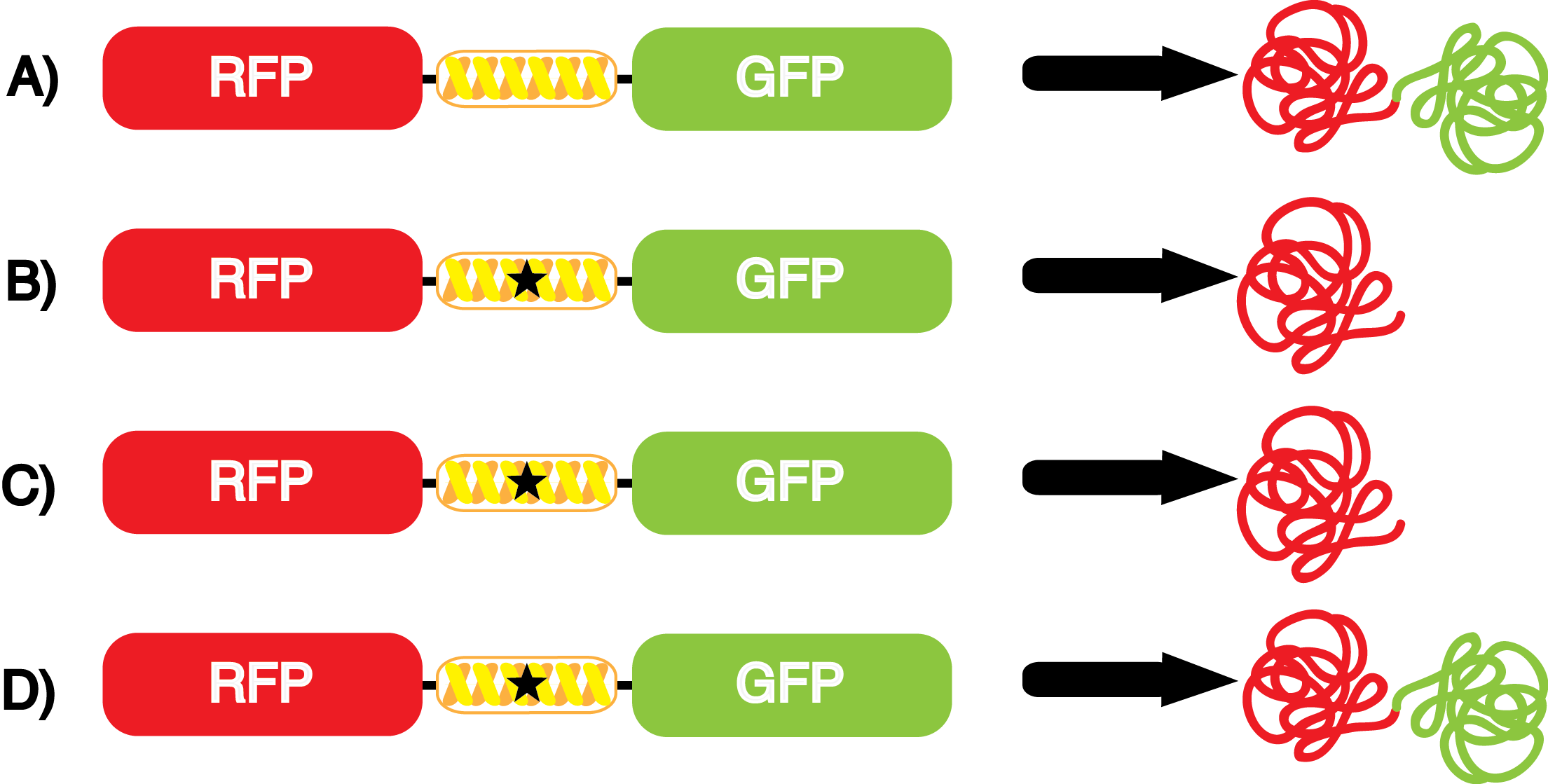
| - | + | Each ncAA synthetase/tRNA pair to be tested was cloned into pStG and transformed into pFRYC amberless ''E. coli'' and pFRY amberless ''E. coli''. Other necessary control strains include RFP amberless ''E. coli'' (RFP control), sfGFP amberless ''E. coli'' (GFP control), amberless ''E. coli'' (cell background control), and LB media supplemented with ncAA (media background control). An overnight culture of each strain was grown in LB with the appropriate antibiotics at 37ºC and 225rpm. 10 mL of media with the appropriate antibiotics was inoculated with 100 µL of overnight culture and allowed to grow in the same conditions until the culture density was ~0.2-0.3 OD<sub>600</sub>, or ~3 hours. The 10 mL culture was split between 4 different sterile test-tubes, 2 mL of culture per tube. The conditions of the four test tubes were as follows: | |
*-IPTG,-ncAA | *-IPTG,-ncAA | ||
*-IPTG,+ncAA | *-IPTG,+ncAA | ||
*+IPTG, -ncAA | *+IPTG, -ncAA | ||
*+IPTG, +ncAA | *+IPTG, +ncAA | ||
| - | If the cultures did not have IPTG or | + | If the cultures did not have IPTG or ncAA, an equal volume of sterile deionized water was added in order to keep the volumes between cultures constant. Once the water, the IPTG, and the ncAA were added appropriately, the cultures were allowed to grow to ~0.5 OD<sub>600</sub>. 70 µL of each culture condition and control culture were added to a separate well in clear-bottom black 96-well plate for fluorescence and OD<sub>600</sub> readings using a plate reader. |
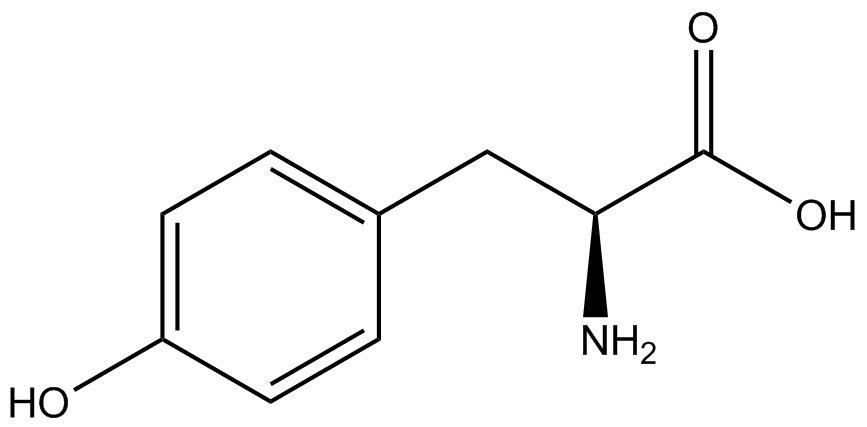
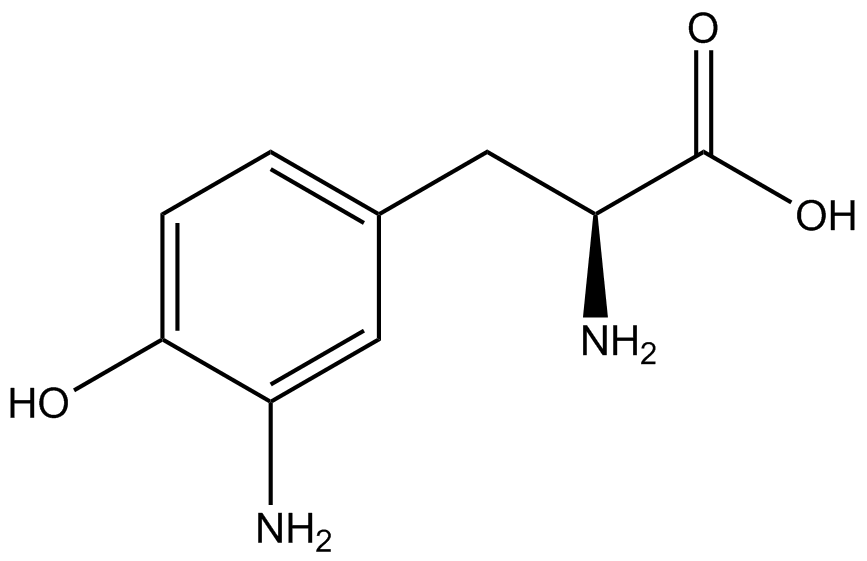
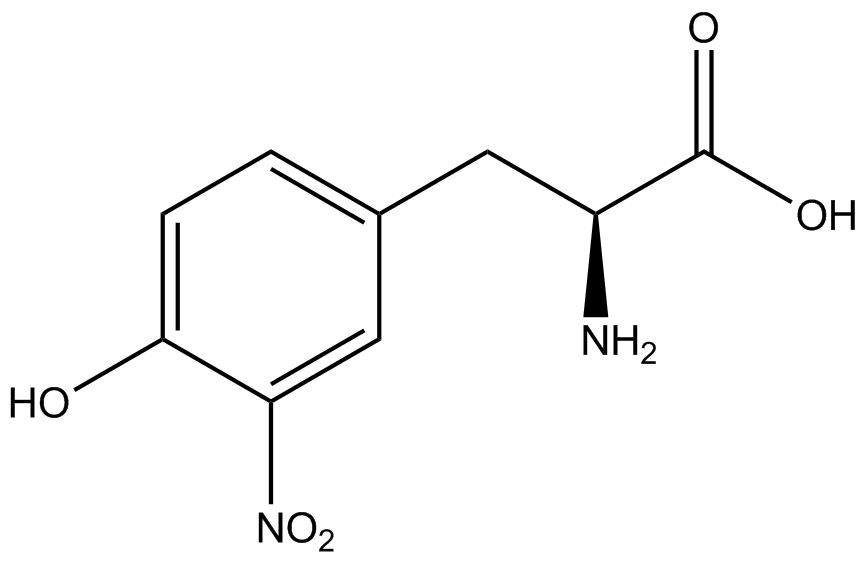
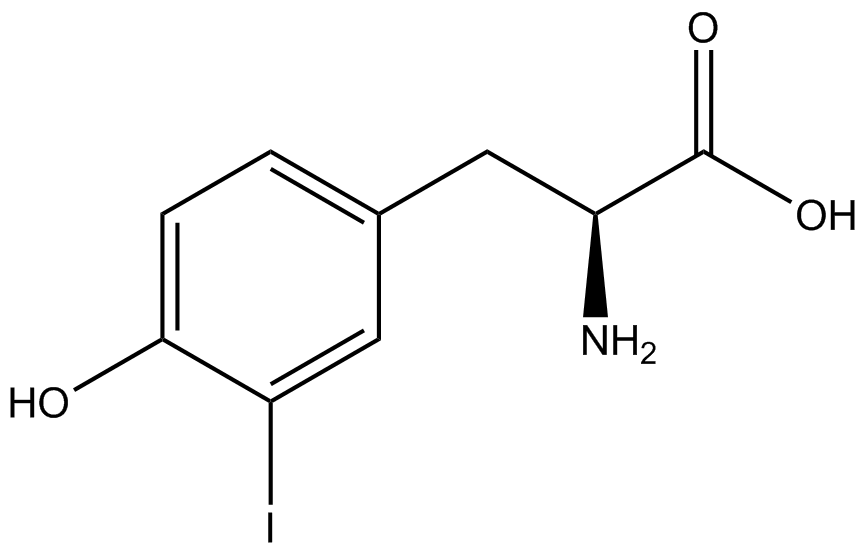
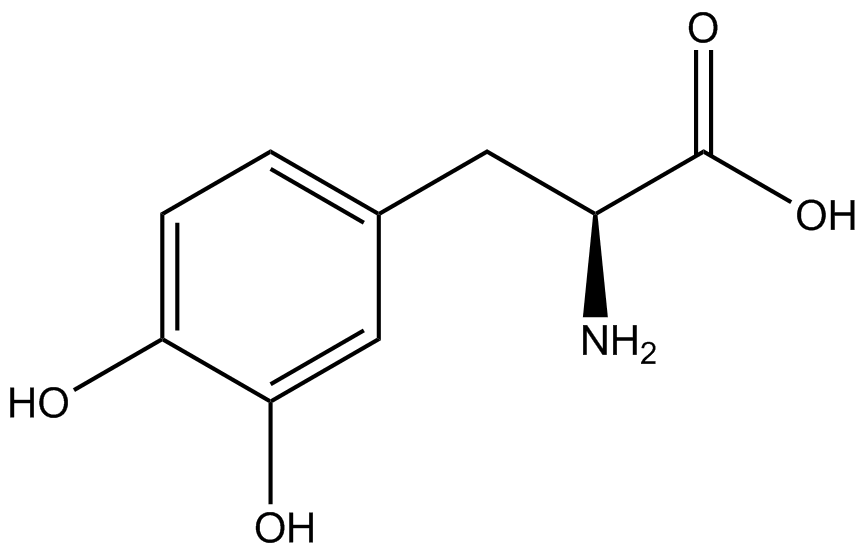
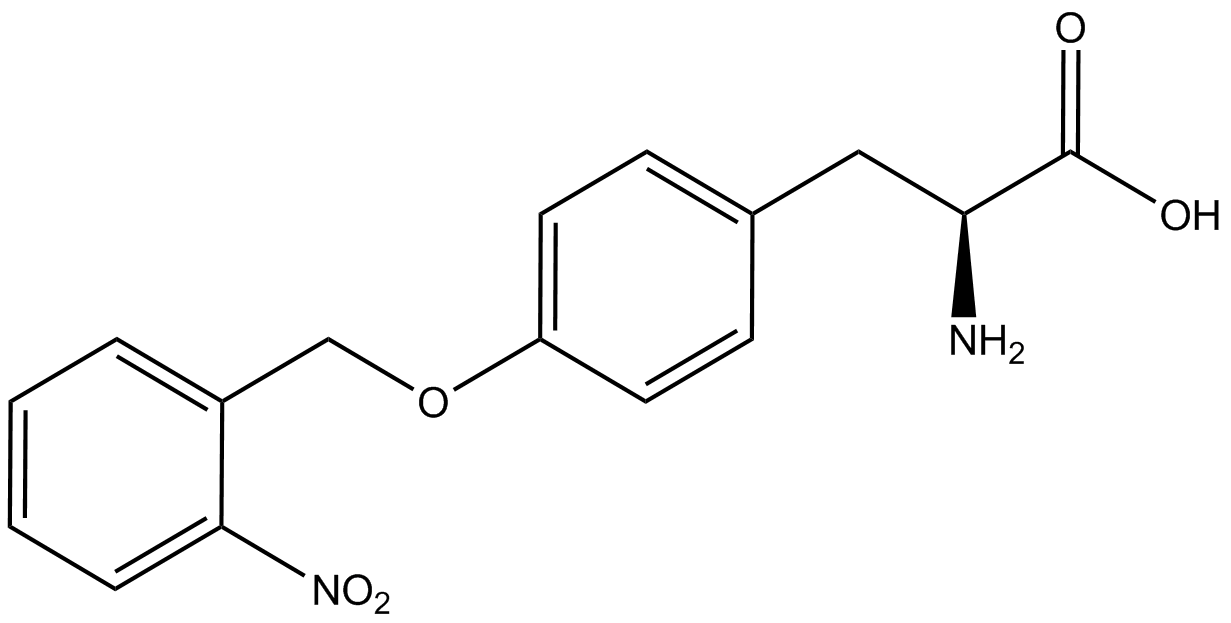
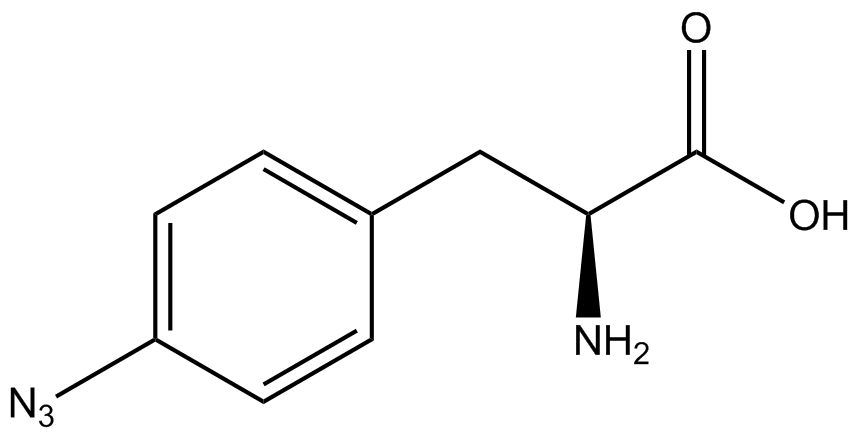
| - | ncAA-specific concerns | + | We accounted for several ncAA-specific concerns. These included: light-sensitivity, oxidation, and interference with fluorescence readings. Certain ncAAs were protected from light due to their light-sensitive molecular structures such as [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table ONBY and AzF]. These ncAAs were prepared in a dark room and wrapped in foil. All cultures were grown in a foil-wrapped incubator for consistency. Some ncAAs are more prone to oxidation, specifically <small>L</small>-DOPA. When oxidized, the solution turns black. To prevent oxidation, each ncAA was prepared the day of the test for consistency. By this method, the oxidation of <small>L</small>-DOPA did not occur quickly enough to have an effect on the data. Most ncAA solutions were transparent once prepared with the exception of 3-nitro-<small>L</small>-tyrosine. The yellow-orange tint of 3-nitro-<small>L</small>-tyrosine solution was accounted for by measuring the fluorescence of 1mM 3-nitro-<small>L</small>-tyrosine in media and subtracting any possible background fluorescence from culture fluorescence grown in 1mM 3-nitro-<small>L</small>-tyrosine. |
Revision as of 02:15, 18 October 2014
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"