Team:Austin Texas/kit
From 2014.igem.org
Jordanmonk (Talk | contribs) m |
|||
| Line 74: | Line 74: | ||
[[file: Austin_Texas_Measurement_Kit_Card.png|right]] | [[file: Austin_Texas_Measurement_Kit_Card.png|right]] | ||
__TOC__ | __TOC__ | ||
| - | + | <div align="justify"> | |
<h1>Kit Introduction</h1> | <h1>Kit Introduction</h1> | ||
In recent years the ability to expand the genetic code has been made possible by re-coding the amber stop codon, UAG, via the use of modified tRNA synthetase/tRNA pairs. These synthetase/tRNA pairs act together to charge the tRNA with a non-canonical amino acid (ncAA), an amino acid that is not one of the 20 amino acids normally encoded by a codon. While the library of ncAA synthetase/tRNA pairs continues to grow, the properties of each pairing have yet to be systematically characterized using a standardized methodology. | In recent years the ability to expand the genetic code has been made possible by re-coding the amber stop codon, UAG, via the use of modified tRNA synthetase/tRNA pairs. These synthetase/tRNA pairs act together to charge the tRNA with a non-canonical amino acid (ncAA), an amino acid that is not one of the 20 amino acids normally encoded by a codon. While the library of ncAA synthetase/tRNA pairs continues to grow, the properties of each pairing have yet to be systematically characterized using a standardized methodology. | ||
| - | |||
There are certain desired properties of a synthetase/tRNA pair, such as high fidelity and high efficiency, that must be characterized. Without knowing these properties, it is impossible to know how effectively a synthetase/tRNA pair will work and the resulting products will remain vague unless specifically analyzed with each use. The 2014 University of Texas at Austin iGEM Team has developed a standardized method that allows for efficient qualitative and quantitative in vivo characterization of ncAA tRNA synthetase/tRNA pairs. This Expanded Genetic Code Measurement Kit is portable, easy-to-use, and can be quickly used with any synthetase/tRNA pair. | There are certain desired properties of a synthetase/tRNA pair, such as high fidelity and high efficiency, that must be characterized. Without knowing these properties, it is impossible to know how effectively a synthetase/tRNA pair will work and the resulting products will remain vague unless specifically analyzed with each use. The 2014 University of Texas at Austin iGEM Team has developed a standardized method that allows for efficient qualitative and quantitative in vivo characterization of ncAA tRNA synthetase/tRNA pairs. This Expanded Genetic Code Measurement Kit is portable, easy-to-use, and can be quickly used with any synthetase/tRNA pair. | ||
| - | |||
| - | |||
<h2>Motivation</h2> | <h2>Motivation</h2> | ||
| Line 100: | Line 97: | ||
The genetic code is a composition of 64 nucleotide triplets (codons) that code for 20 highly conserved amino acids that are essential to all organisms on Earth. While the genetic code is specific, it is also degenerate, meaning that more than one codon can encode for the incorporation of a specific amino acid. For example, there are six serine codons and three stop codons (called amber, ochre, and opal). By recoding one of the redundant codons, the recoded codon can signal for the incorporation of a non-canonical amino acid (ncAA) rather than the codon's original usage. Of the three stop codons, the amber codon is the least abundant and thus, the easiest and most efficient to recode. | The genetic code is a composition of 64 nucleotide triplets (codons) that code for 20 highly conserved amino acids that are essential to all organisms on Earth. While the genetic code is specific, it is also degenerate, meaning that more than one codon can encode for the incorporation of a specific amino acid. For example, there are six serine codons and three stop codons (called amber, ochre, and opal). By recoding one of the redundant codons, the recoded codon can signal for the incorporation of a non-canonical amino acid (ncAA) rather than the codon's original usage. Of the three stop codons, the amber codon is the least abundant and thus, the easiest and most efficient to recode. | ||
| - | |||
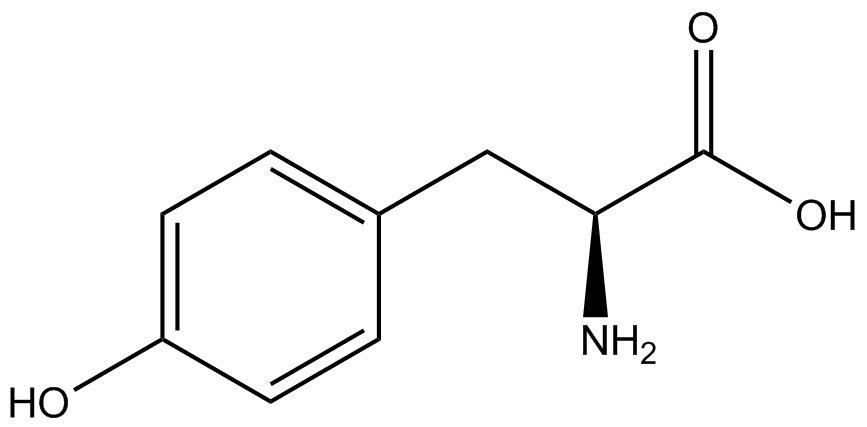
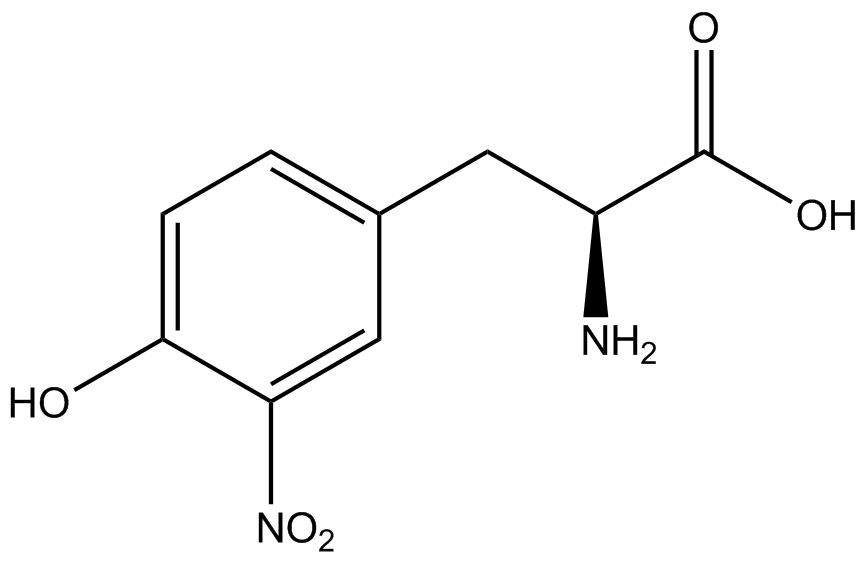
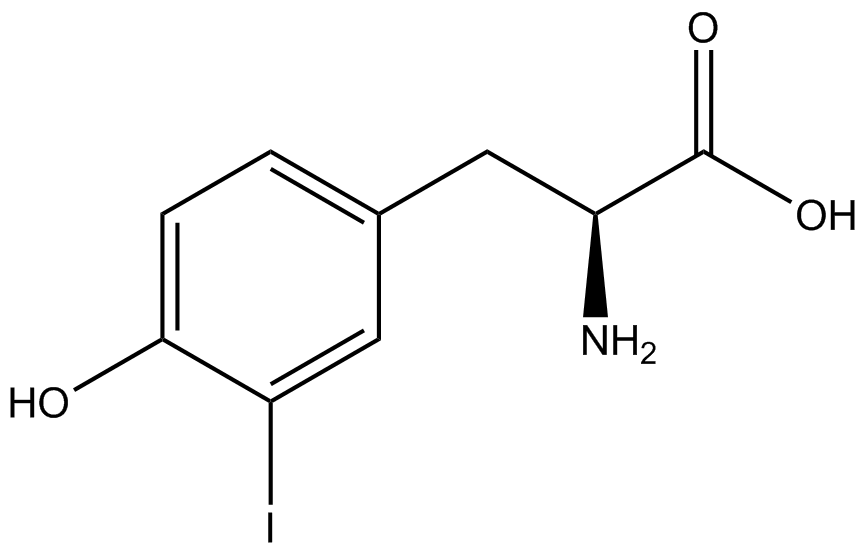
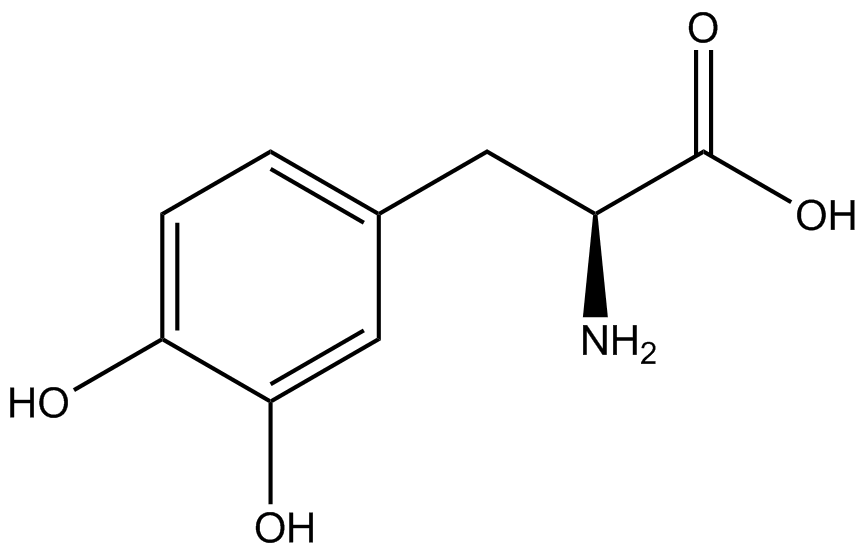
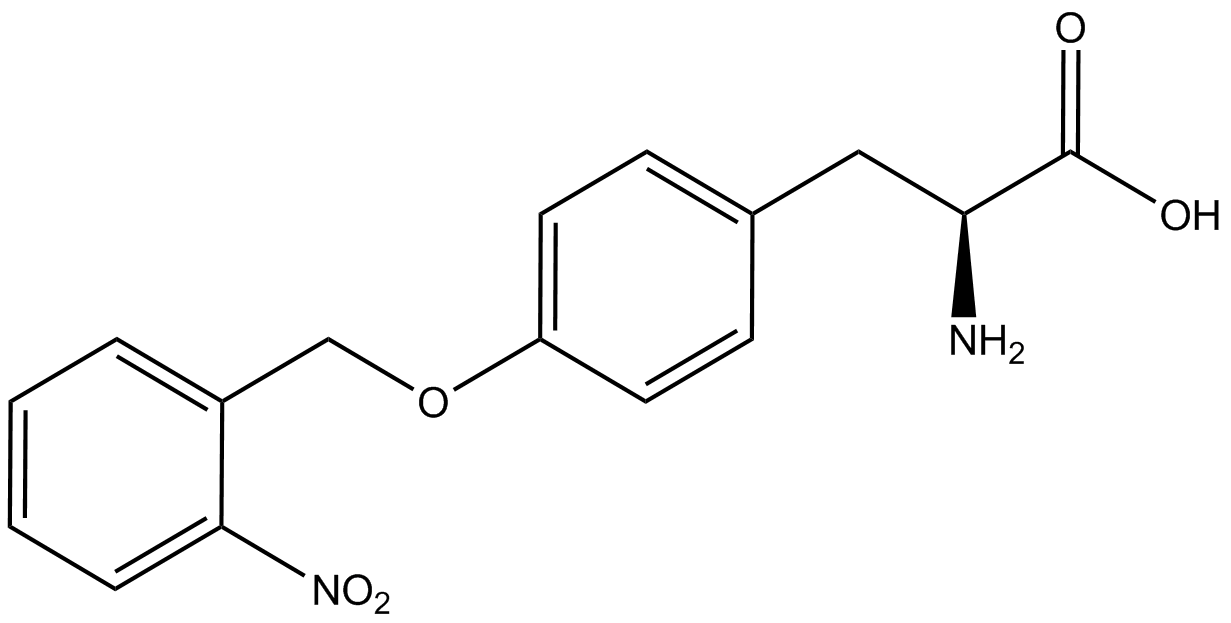
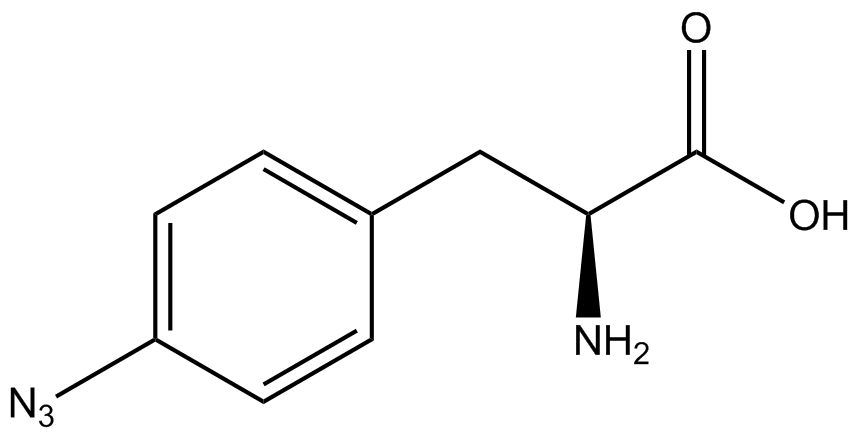
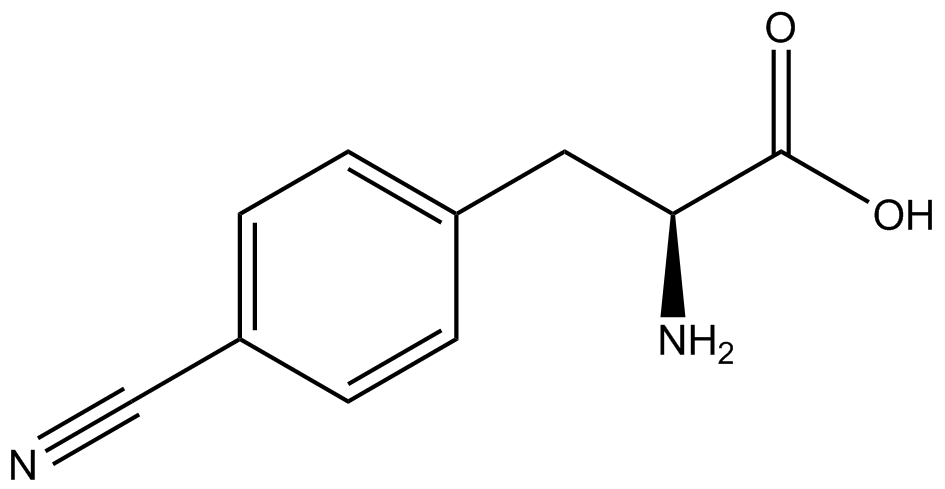
The Schultz lab was the first to expand the genetic code using a unique synthetase/tRNA pair. This synthetase/tRNA pair originated from <i>M. jannaschii</i> tyrosine RS (Mj-TyrRS) and allowed the cell to incorporate <i>o</i>-methyl-<small>L</small>-tyrosine at the amber codon (Wang et al. 2001). Since then, numerous ncAA synthetase/tRNA pairs have been generated. A total of seven ncAAs were used in addition to tyrosine for our project. [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table The full list of amino acids used in this study can be found here]. | The Schultz lab was the first to expand the genetic code using a unique synthetase/tRNA pair. This synthetase/tRNA pair originated from <i>M. jannaschii</i> tyrosine RS (Mj-TyrRS) and allowed the cell to incorporate <i>o</i>-methyl-<small>L</small>-tyrosine at the amber codon (Wang et al. 2001). Since then, numerous ncAA synthetase/tRNA pairs have been generated. A total of seven ncAAs were used in addition to tyrosine for our project. [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table The full list of amino acids used in this study can be found here]. | ||
| - | |||
It is important to note that complications can arise when the genetic code is recoded. In a normal bacterium, release factor RF1 is responsible for terminating translation when the ribosome reaches an amber stop codon. To expand the potential of ncAAs, it is vital to avoid termination at amber codons. Seeing this need, the Church and Isaacs groups engineered a strain of ''E. coli'' that had all of the amber codons removed from the genome, which allowed them to knock out the RF1 gene (Isaacs et al. 2011). The resulting strain, called "amberless" ''E. coli'', has NO AMBER CODONS. Thus, to better characterize the synthetase/tRNA pairs, we have employed amberless ''E. coli''. The advantage of this system is that since there are no amber codons in the genome, we cannot accidentally interfere with any cellular processes. Additionally, since RF1 is knocked out, there is no competition between RF1 and the novel synthetase/tRNA pair. These factors allow us to better assess the fidelity and efficiency of ncAA tRNA synthetase/tRNA pairs. | It is important to note that complications can arise when the genetic code is recoded. In a normal bacterium, release factor RF1 is responsible for terminating translation when the ribosome reaches an amber stop codon. To expand the potential of ncAAs, it is vital to avoid termination at amber codons. Seeing this need, the Church and Isaacs groups engineered a strain of ''E. coli'' that had all of the amber codons removed from the genome, which allowed them to knock out the RF1 gene (Isaacs et al. 2011). The resulting strain, called "amberless" ''E. coli'', has NO AMBER CODONS. Thus, to better characterize the synthetase/tRNA pairs, we have employed amberless ''E. coli''. The advantage of this system is that since there are no amber codons in the genome, we cannot accidentally interfere with any cellular processes. Additionally, since RF1 is knocked out, there is no competition between RF1 and the novel synthetase/tRNA pair. These factors allow us to better assess the fidelity and efficiency of ncAA tRNA synthetase/tRNA pairs. | ||
| Line 113: | Line 108: | ||
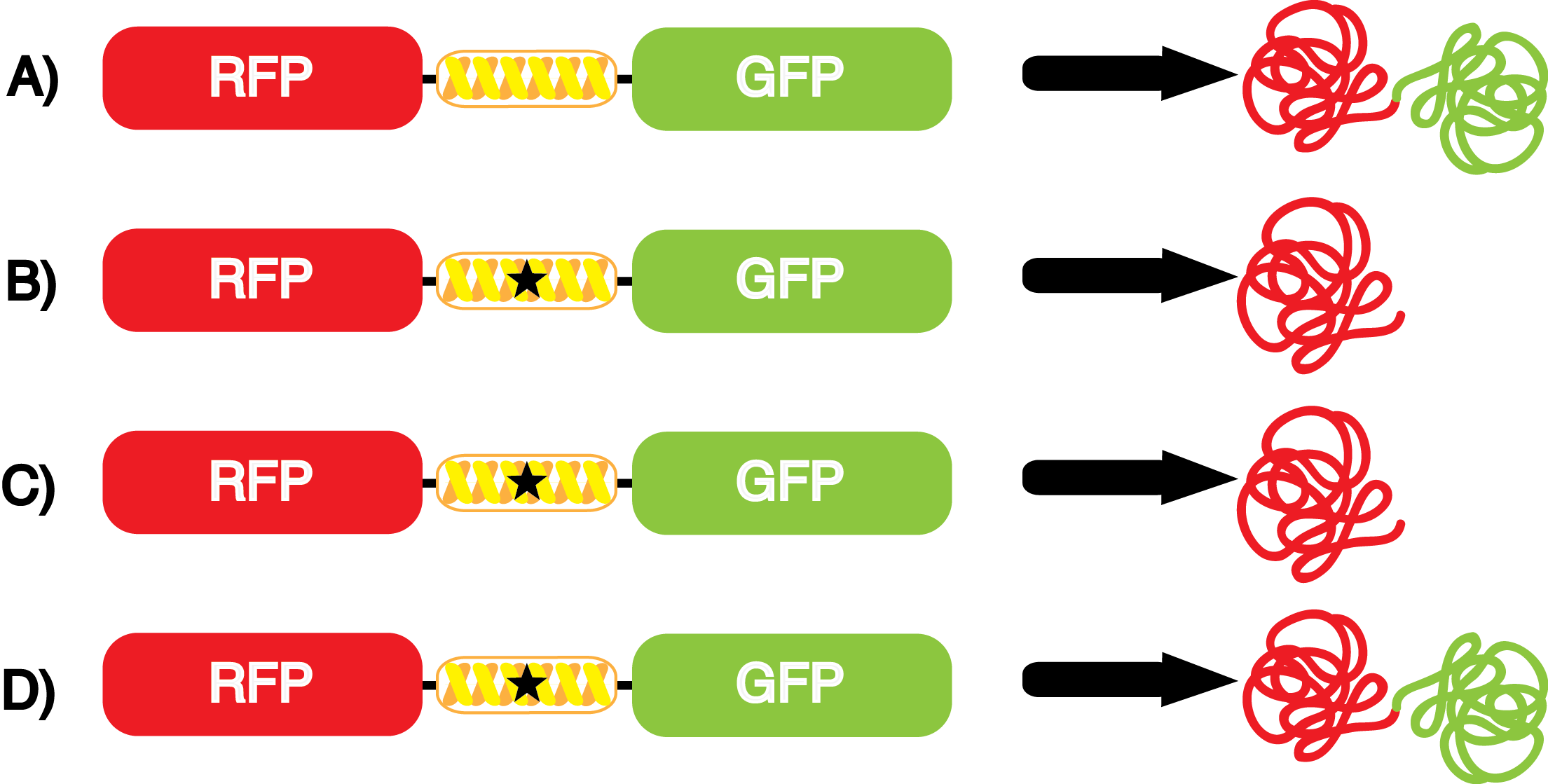
[[File:Kit Plasmid Alignment 10-14-14.png|570px|thumb|right|<b>Figure 2.</b> | [[File:Kit Plasmid Alignment 10-14-14.png|570px|thumb|right|<b>Figure 2.</b> | ||
pStG contains the specific ncAA synthetase/tRNA pair being tested. pFRYC and pFRY are the ncAA kit reporter plasmids. pFRYC is the control plasmid that yields GFP and RFP expression regardless of synthetase/tRNA pair or the presence of ncAA. pFRY is a nearly identical plasmid with a single difference, the linker between the GFP and RFP sequences contains an amber codon (star) in place of the tyrosine codon found in pFRYC. Thus, the level of GFP expression for pFRY is directly dependent on the efficiency and fidelity of the synthetase/tRNA pair being tested.]] | pStG contains the specific ncAA synthetase/tRNA pair being tested. pFRYC and pFRY are the ncAA kit reporter plasmids. pFRYC is the control plasmid that yields GFP and RFP expression regardless of synthetase/tRNA pair or the presence of ncAA. pFRY is a nearly identical plasmid with a single difference, the linker between the GFP and RFP sequences contains an amber codon (star) in place of the tyrosine codon found in pFRYC. Thus, the level of GFP expression for pFRY is directly dependent on the efficiency and fidelity of the synthetase/tRNA pair being tested.]] | ||
| - | |||
The kit consists of a three plasmid system: pStG, pFRYC, and pFRY ('''Figure 2'''). | The kit consists of a three plasmid system: pStG, pFRYC, and pFRY ('''Figure 2'''). | ||
| Line 143: | Line 137: | ||
*''' D)''' In a cell containing pStG and pFRY, there are multiple possible outcomes. In the PRESENCE of ncAA, the ribosome will translate the RFP, then it incorporates the ncAA at the amber codon in the linker, and finally it proceeds to translate the downstream sfGFP reporter, producing a visible yellow fluorescence (Figure 3D). This occurs because the synthetase found in pStG can covalently attach the ncAA to the tRNA gene present in pStG. This "charged tRNA" is then present during translation, allowing incorporation of the ncAA into the reporter protein. | *''' D)''' In a cell containing pStG and pFRY, there are multiple possible outcomes. In the PRESENCE of ncAA, the ribosome will translate the RFP, then it incorporates the ncAA at the amber codon in the linker, and finally it proceeds to translate the downstream sfGFP reporter, producing a visible yellow fluorescence (Figure 3D). This occurs because the synthetase found in pStG can covalently attach the ncAA to the tRNA gene present in pStG. This "charged tRNA" is then present during translation, allowing incorporation of the ncAA into the reporter protein. | ||
| - | |||
However, the above scenarios assume that the synthetase/tRNA pair function efficiently and with high fidelity. | However, the above scenarios assume that the synthetase/tRNA pair function efficiently and with high fidelity. | ||
| Line 151: | Line 144: | ||
<h2>Experimental Preparation</h2> | <h2>Experimental Preparation</h2> | ||
| - | |||
| - | |||
[[File:Alex and Foil-covered incubator.jpg|200px|thumb|right|Protection of light-sensitive ncAAs using a foil-wrapped incubator.]] | [[File:Alex and Foil-covered incubator.jpg|200px|thumb|right|Protection of light-sensitive ncAAs using a foil-wrapped incubator.]] | ||
| Line 161: | Line 152: | ||
*+IPTG, +ncAA | *+IPTG, +ncAA | ||
If the cultures did not have IPTG or an ncAA, an equal volume of sterile deionized water was added in order to keep the volumes between cultures constant. Once the water, the IPTG, and the ncAA were added appropriately, the cultures were allowed to grow to ~0.5 OD<sub>600</sub>. 70 µL of each culture condition and control culture were added to a separate well in clear-bottom black 96-well plate for fluorescence and OD<sub>600</sub> readings using a plate reader. | If the cultures did not have IPTG or an ncAA, an equal volume of sterile deionized water was added in order to keep the volumes between cultures constant. Once the water, the IPTG, and the ncAA were added appropriately, the cultures were allowed to grow to ~0.5 OD<sub>600</sub>. 70 µL of each culture condition and control culture were added to a separate well in clear-bottom black 96-well plate for fluorescence and OD<sub>600</sub> readings using a plate reader. | ||
| - | |||
ncAA-specific concerns accounted for and should be noted. These include: light-sensitivity, oxidation, and interference with fluorescence readings. Certain ncAAs were protected from light due to their light-sensitive molecular structures such as [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table ONBY and AzF]. These ncAAs were prepared in a dark room and wrapped in foil. All cultures were grown in a foil-wrapped incubator for consistency. Some ncAAs are more prone to oxidation, specifically <small>L</small>-DOPA. When oxidized, the solution turns black. To prevent oxidation, each ncAA was prepared the day of the test for consistency. By this method, the oxidation of <small>L</small>-DOPA did not occur quickly enough to have an effect on the data. Most ncAA solutions were transparent once prepared with the exception of 3-nitro-<small>L</small>-tyrosine. The yellow-orange tint of 3-nitro-<small>L</small>-tyrosine solution was accounted for by measuring the fluorescence of 1mM 3-nitro-<small>L</small>-tyrosine in media and subtracting any possible background fluorescence from culture fluorescence grown in 1mM 3-nitro-<small>L</small>-tyrosine. | ncAA-specific concerns accounted for and should be noted. These include: light-sensitivity, oxidation, and interference with fluorescence readings. Certain ncAAs were protected from light due to their light-sensitive molecular structures such as [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table ONBY and AzF]. These ncAAs were prepared in a dark room and wrapped in foil. All cultures were grown in a foil-wrapped incubator for consistency. Some ncAAs are more prone to oxidation, specifically <small>L</small>-DOPA. When oxidized, the solution turns black. To prevent oxidation, each ncAA was prepared the day of the test for consistency. By this method, the oxidation of <small>L</small>-DOPA did not occur quickly enough to have an effect on the data. Most ncAA solutions were transparent once prepared with the exception of 3-nitro-<small>L</small>-tyrosine. The yellow-orange tint of 3-nitro-<small>L</small>-tyrosine solution was accounted for by measuring the fluorescence of 1mM 3-nitro-<small>L</small>-tyrosine in media and subtracting any possible background fluorescence from culture fluorescence grown in 1mM 3-nitro-<small>L</small>-tyrosine. | ||
| Line 200: | Line 190: | ||
We wanted to test whether the ncAA synthetase/tRNA pairs would incorporate anything besides their amino acid at the amber stop codon (UAG), and that they would in fact incorporate their specific ncAA if it was present. This is essentially the fidelity of each ncAA sythetase/tRNA pair. | We wanted to test whether the ncAA synthetase/tRNA pairs would incorporate anything besides their amino acid at the amber stop codon (UAG), and that they would in fact incorporate their specific ncAA if it was present. This is essentially the fidelity of each ncAA sythetase/tRNA pair. | ||
| - | |||
Fidelity was measured by comparing the production of GFP in cultures containing pStG/pFRY with or without ncAA. In the absence of a ncAA only RFP should be translated, as translation is expected to halt between RFP and GFP at the amber codon on the linker sequence in pFRY. Alternatively, if the corresponding ncAA for pStG is present or if the synthetase/tRNA pair has low fidelity and can misincorporate a different amino acid, translation should continue through the UAG. In this case, RFP and GFP should both be translated. We also tested pStG/pFRYC strains in (+/-) ncAA conditions as a control for what effect the ncAA has on the fluorescence or growth of the cells. These strains should express RFP and GFP in all conditions since pFRYC does not have an amber codon in the linker (Figure 2). However, if the presence of ncAA affects cell growth or fluorescence activity, we will need these controls to determine the extent of the effect. | Fidelity was measured by comparing the production of GFP in cultures containing pStG/pFRY with or without ncAA. In the absence of a ncAA only RFP should be translated, as translation is expected to halt between RFP and GFP at the amber codon on the linker sequence in pFRY. Alternatively, if the corresponding ncAA for pStG is present or if the synthetase/tRNA pair has low fidelity and can misincorporate a different amino acid, translation should continue through the UAG. In this case, RFP and GFP should both be translated. We also tested pStG/pFRYC strains in (+/-) ncAA conditions as a control for what effect the ncAA has on the fluorescence or growth of the cells. These strains should express RFP and GFP in all conditions since pFRYC does not have an amber codon in the linker (Figure 2). However, if the presence of ncAA affects cell growth or fluorescence activity, we will need these controls to determine the extent of the effect. | ||
| - | |||
To determine the change in GFP fluorescence when the ncAA was present, we first had to calculate how much GFP was expressed relative to the RFP, which would give an upper estimate of how much GFP could theoretically be expressed. We first divided both the GFP and RFP levels by the OD<sub>600</sub> of the culture in order to get the per cell fluorescence levels. We then normalized the GFP fluorescence for one culture to its RFP fluorescence so that we could compare the GFP fluorescence levels between cultures. The normalized GFP values were then compared between cultures grown in the presence of ncAA and cultures grown in the absence of ncAA, which would indicate how the level of GFP fluorescence changes when the ncAA is present. | To determine the change in GFP fluorescence when the ncAA was present, we first had to calculate how much GFP was expressed relative to the RFP, which would give an upper estimate of how much GFP could theoretically be expressed. We first divided both the GFP and RFP levels by the OD<sub>600</sub> of the culture in order to get the per cell fluorescence levels. We then normalized the GFP fluorescence for one culture to its RFP fluorescence so that we could compare the GFP fluorescence levels between cultures. The normalized GFP values were then compared between cultures grown in the presence of ncAA and cultures grown in the absence of ncAA, which would indicate how the level of GFP fluorescence changes when the ncAA is present. | ||
| - | |||
When these values were graphed (Figure 4), some synthetase/tRNA pairs such as 4-azido-<small>L</small>-phenylalanine (AzF), 3-nitro-<small>L</small>-tyrosine, 3-iodo-<small>L</small>-tyrosine, and ''o''-(2-nitrobenzyl)-<small>L</small>-tyrosine (ONBY) resulted in higher GFP fluorescence in the presence of ncAA than in the absence of ncAA, which suggests that those synthetases only incorporated an amino acid if their specific amino acid was present, meaning that they have a high fidelity. However, the other synthetase/tRNA pairs (3-amino-<small>L</small>-tyrosine, <small>L</small>-DOPA, and 4-cyano-<small>L</small>-phenylalanine (CNF)) did not show a difference in GFP fluorescence normalized to RFP fluorescence dependent on the presence of ncAA, indicating that these pairs incorporated other amino acids at the amber codon when their specific ncAA was not present (and perhaps even when it was), and thus have a low fidelity. | When these values were graphed (Figure 4), some synthetase/tRNA pairs such as 4-azido-<small>L</small>-phenylalanine (AzF), 3-nitro-<small>L</small>-tyrosine, 3-iodo-<small>L</small>-tyrosine, and ''o''-(2-nitrobenzyl)-<small>L</small>-tyrosine (ONBY) resulted in higher GFP fluorescence in the presence of ncAA than in the absence of ncAA, which suggests that those synthetases only incorporated an amino acid if their specific amino acid was present, meaning that they have a high fidelity. However, the other synthetase/tRNA pairs (3-amino-<small>L</small>-tyrosine, <small>L</small>-DOPA, and 4-cyano-<small>L</small>-phenylalanine (CNF)) did not show a difference in GFP fluorescence normalized to RFP fluorescence dependent on the presence of ncAA, indicating that these pairs incorporated other amino acids at the amber codon when their specific ncAA was not present (and perhaps even when it was), and thus have a low fidelity. | ||
| Line 213: | Line 200: | ||
Another measure of quality for these ncAA synthetase/tRNA pairs is how efficiently they charge their ncAA, ultimately resulting in incorporation of the ncAA in our reporter protein. An inefficient synthetase/tRNA pair will yield incorporation of their ncAA only a fraction of the time, even when their ncAA is present. Our system can be used to measure this level of efficiency, though it does not indicate why a particular synthetase is efficient or inefficient. By comparing the level of GFP fluorescence to the normalized level of RFP fluorescence when the amino acid is present, we can see how efficient the synthetase is. In essence, if the normalized fluorescence of GFP relative to RFP is close to 100% (if the GFP is expressed roughly 100% of the time that RFP is expressed), then the synthetase is very efficient. On the other side, if say the normalized fluorescence of GFP relative to RFP is closer to 10%, then the synthetase would not be very efficient, because even when the ncAA was there, it only incorporated it at the amber codon about 10% of the time. | Another measure of quality for these ncAA synthetase/tRNA pairs is how efficiently they charge their ncAA, ultimately resulting in incorporation of the ncAA in our reporter protein. An inefficient synthetase/tRNA pair will yield incorporation of their ncAA only a fraction of the time, even when their ncAA is present. Our system can be used to measure this level of efficiency, though it does not indicate why a particular synthetase is efficient or inefficient. By comparing the level of GFP fluorescence to the normalized level of RFP fluorescence when the amino acid is present, we can see how efficient the synthetase is. In essence, if the normalized fluorescence of GFP relative to RFP is close to 100% (if the GFP is expressed roughly 100% of the time that RFP is expressed), then the synthetase is very efficient. On the other side, if say the normalized fluorescence of GFP relative to RFP is closer to 10%, then the synthetase would not be very efficient, because even when the ncAA was there, it only incorporated it at the amber codon about 10% of the time. | ||
| - | |||
For our results (Figure 4), two synthetase/tRNA pairs stood out as relatively inefficient: 3-nitrotyrosine and ONBY. Both of these synthetase/tRNA pairs showed a significantly smaller normalized GFP to RFP fluorescence when the ncAA was present with the pFRY construct. While both synthetase/tRNA pairs show a significant increase in normalized GFP to RFP fluorescence when the amino acid was present compared to when it was absent, which indicates a high fidelity, the actual GFP fluorescence relative to the RFP fluorescence was only around 50% for 3-nitrotyrosine and 20% for ONBY. These results suggest that, these synthetase/tRNA pairs do not always incorporate their ncAA at the amber stop codon. It is important to note that this could partly result for ncAA-related toxicity, and such an effect was repeatedly observed for ONBY. | For our results (Figure 4), two synthetase/tRNA pairs stood out as relatively inefficient: 3-nitrotyrosine and ONBY. Both of these synthetase/tRNA pairs showed a significantly smaller normalized GFP to RFP fluorescence when the ncAA was present with the pFRY construct. While both synthetase/tRNA pairs show a significant increase in normalized GFP to RFP fluorescence when the amino acid was present compared to when it was absent, which indicates a high fidelity, the actual GFP fluorescence relative to the RFP fluorescence was only around 50% for 3-nitrotyrosine and 20% for ONBY. These results suggest that, these synthetase/tRNA pairs do not always incorporate their ncAA at the amber stop codon. It is important to note that this could partly result for ncAA-related toxicity, and such an effect was repeatedly observed for ONBY. | ||
| Line 230: | Line 216: | ||
<h1>Discussion</h1> | <h1>Discussion</h1> | ||
Our ncAA measurement kit was able to successfully compare the fidelity and efficiency of seven different synthetase/tRNA pairs. We were able to confidently say that one synthetase/tRNA pair had a higher fidelity than another. We saw clear evidence that three of our tRNA synthetase/tRNA pairs seemed incapable of discriminating between their ncAA and the 20 canonical amino acids present in media: 3-aminotyrosine, <small>L</small>-DOPA, and CNF. We were able to test triplicate cultures of seven different ncAAs and tyrosine (eight total synthetase/tRNA pairs) in one day, generating results that immediately told us which ncAAs worked best in our system both in terms of fidelity and efficiency of incorporation. This required no advanced training or access to equipment besides a shaker and a fluorometer. | Our ncAA measurement kit was able to successfully compare the fidelity and efficiency of seven different synthetase/tRNA pairs. We were able to confidently say that one synthetase/tRNA pair had a higher fidelity than another. We saw clear evidence that three of our tRNA synthetase/tRNA pairs seemed incapable of discriminating between their ncAA and the 20 canonical amino acids present in media: 3-aminotyrosine, <small>L</small>-DOPA, and CNF. We were able to test triplicate cultures of seven different ncAAs and tyrosine (eight total synthetase/tRNA pairs) in one day, generating results that immediately told us which ncAAs worked best in our system both in terms of fidelity and efficiency of incorporation. This required no advanced training or access to equipment besides a shaker and a fluorometer. | ||
| - | |||
While the measurements may not be perfectly accurate due to the nature of this test, the measurement kit is designed more around ease of use, cost-efficiency, and portability. The ncAA measurement kit can be used to quickly test a synthetase/tRNA pair's quality with minimal effort, as opposed to other more accurate but more intensive or expensive measures such as mass spectrometry. While mass spectrometry can give much more definitive information about a synthetase/tRNA pair, there are many different factors that could make it unreasonable for undergraduate research. It is a technique that requires a fair amount of skill to do properly, and can be very costly as well. Additionally, the sample needs to be purified from cell culture and prepped for mass spectrometry. | While the measurements may not be perfectly accurate due to the nature of this test, the measurement kit is designed more around ease of use, cost-efficiency, and portability. The ncAA measurement kit can be used to quickly test a synthetase/tRNA pair's quality with minimal effort, as opposed to other more accurate but more intensive or expensive measures such as mass spectrometry. While mass spectrometry can give much more definitive information about a synthetase/tRNA pair, there are many different factors that could make it unreasonable for undergraduate research. It is a technique that requires a fair amount of skill to do properly, and can be very costly as well. Additionally, the sample needs to be purified from cell culture and prepped for mass spectrometry. | ||
| - | |||
For the ncAA measurement kit, all that is necessary is to request from us the kit plasmids (pFRYC and PFRY in separate strains of amberless ''E. coli''), which we are immediately making available to anyone who requests the Expanded Genetic Code Measurement Kit. Once you receive the frozen cultures (or plasmids), transform the synthetase/tRNA pair that you wish to test into the two different cell strains, and run the experiment. Thus, if you receive the measurement kit on Monday, you could have your synthetase/tRNA pairs characterized by Friday. The kit can also be used to test how one or more synthetase/tRNA pairs work in different cells or under different conditions. However, in this case, you may need to retransform the kit plasmids into the different strain. | For the ncAA measurement kit, all that is necessary is to request from us the kit plasmids (pFRYC and PFRY in separate strains of amberless ''E. coli''), which we are immediately making available to anyone who requests the Expanded Genetic Code Measurement Kit. Once you receive the frozen cultures (or plasmids), transform the synthetase/tRNA pair that you wish to test into the two different cell strains, and run the experiment. Thus, if you receive the measurement kit on Monday, you could have your synthetase/tRNA pairs characterized by Friday. The kit can also be used to test how one or more synthetase/tRNA pairs work in different cells or under different conditions. However, in this case, you may need to retransform the kit plasmids into the different strain. | ||
We are optimistic that this measurement kit can help speed up the process of developing a new synthetase/tRNA pair, as well as characterizing large groups of synthetase/tRNA pairs, as we now have a way to quickly, cheaply, and easily do large scale characterizations. | We are optimistic that this measurement kit can help speed up the process of developing a new synthetase/tRNA pair, as well as characterizing large groups of synthetase/tRNA pairs, as we now have a way to quickly, cheaply, and easily do large scale characterizations. | ||
| - | |||
Due to the nature of the Expanded Genetic Code Measurement Kit, <i>in vivo</i> influences will affect certain data and results. This knowledge is very useful by providing a broader and biological perspective of <i>in vivo</i> perspective of cellular health. In general, the presence of ncAAs slowed the growth of amberless ''E. coli'', sometimes quite significantly. This knowledge, as seen through the GFP:RFP ratio, can be very informative when assessing whether to use a ncAA tRNA synthetase/tRNA pair. | Due to the nature of the Expanded Genetic Code Measurement Kit, <i>in vivo</i> influences will affect certain data and results. This knowledge is very useful by providing a broader and biological perspective of <i>in vivo</i> perspective of cellular health. In general, the presence of ncAAs slowed the growth of amberless ''E. coli'', sometimes quite significantly. This knowledge, as seen through the GFP:RFP ratio, can be very informative when assessing whether to use a ncAA tRNA synthetase/tRNA pair. | ||
| - | |||
<h1>Conclusion</h1> | <h1>Conclusion</h1> | ||
| Line 249: | Line 231: | ||
Information regarding the ncAAs used can also be found [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table below]. | Information regarding the ncAAs used can also be found [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table below]. | ||
| - | + | </div> | |
<h1>ncAA Table</h1> | <h1>ncAA Table</h1> | ||
| - | |||
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 01:53, 18 October 2014
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"