Team:Austin Texas/kit
From 2014.igem.org
(→Motivation) |
(→Synthetase efficiency) |
||
| Line 196: | Line 196: | ||
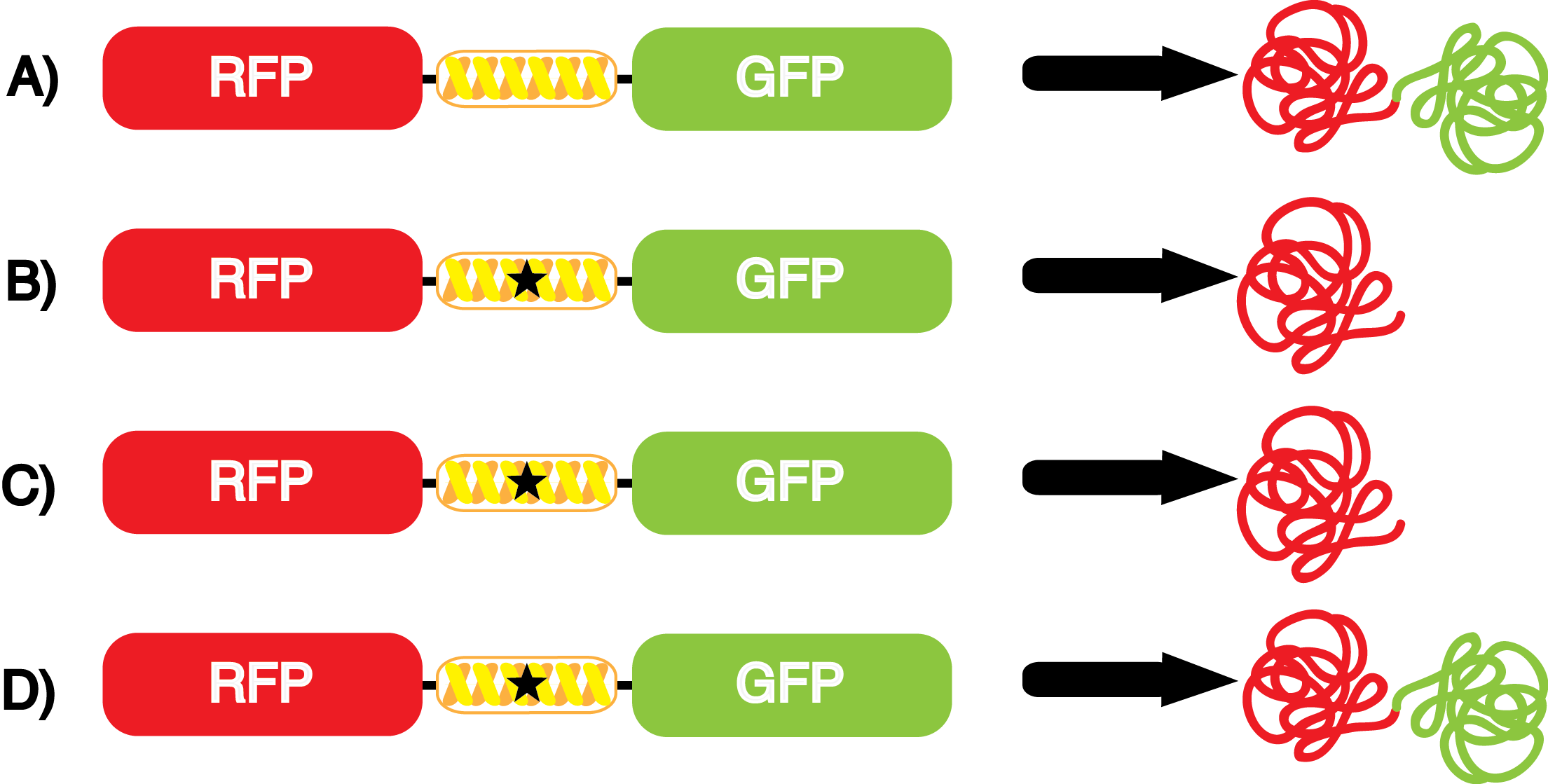
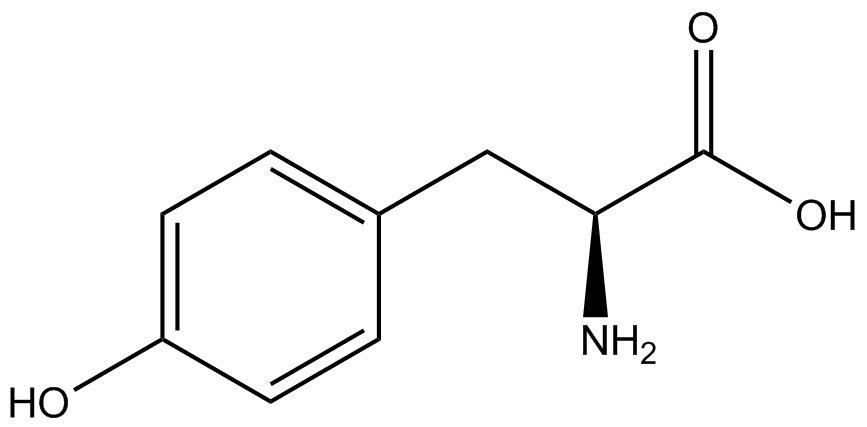
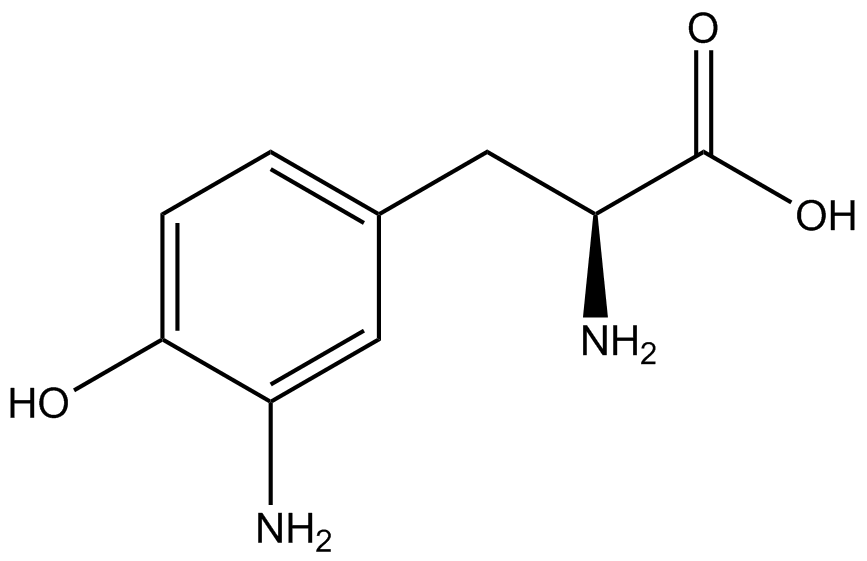
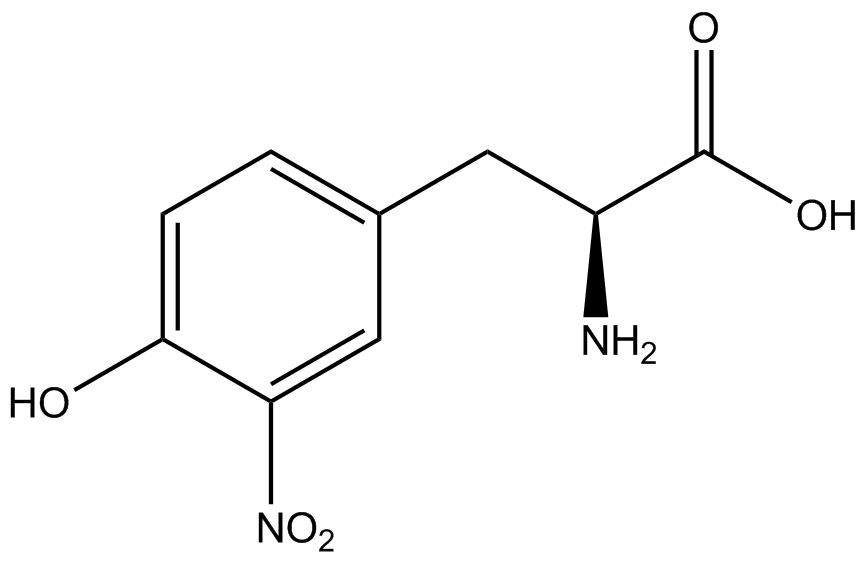
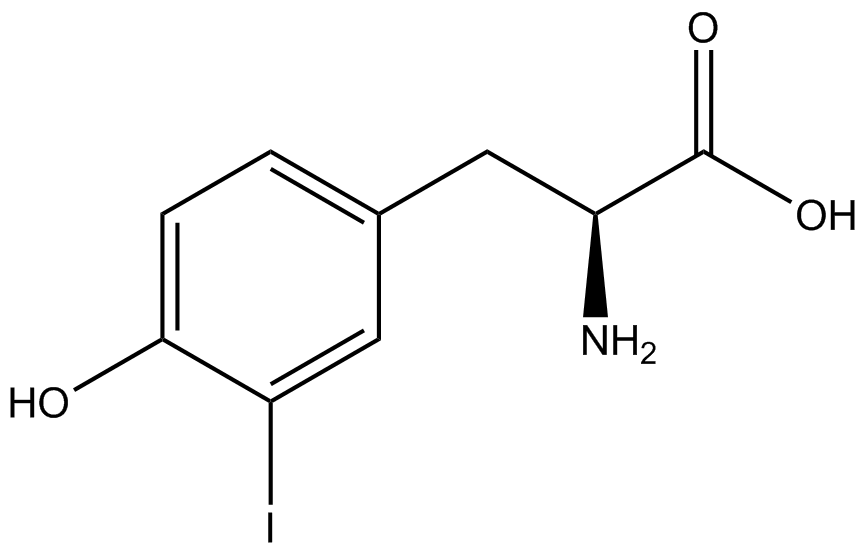
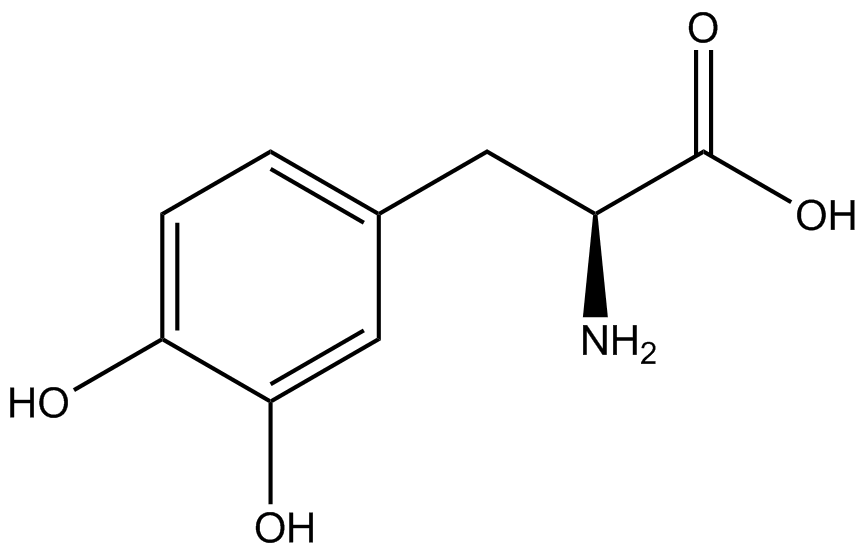
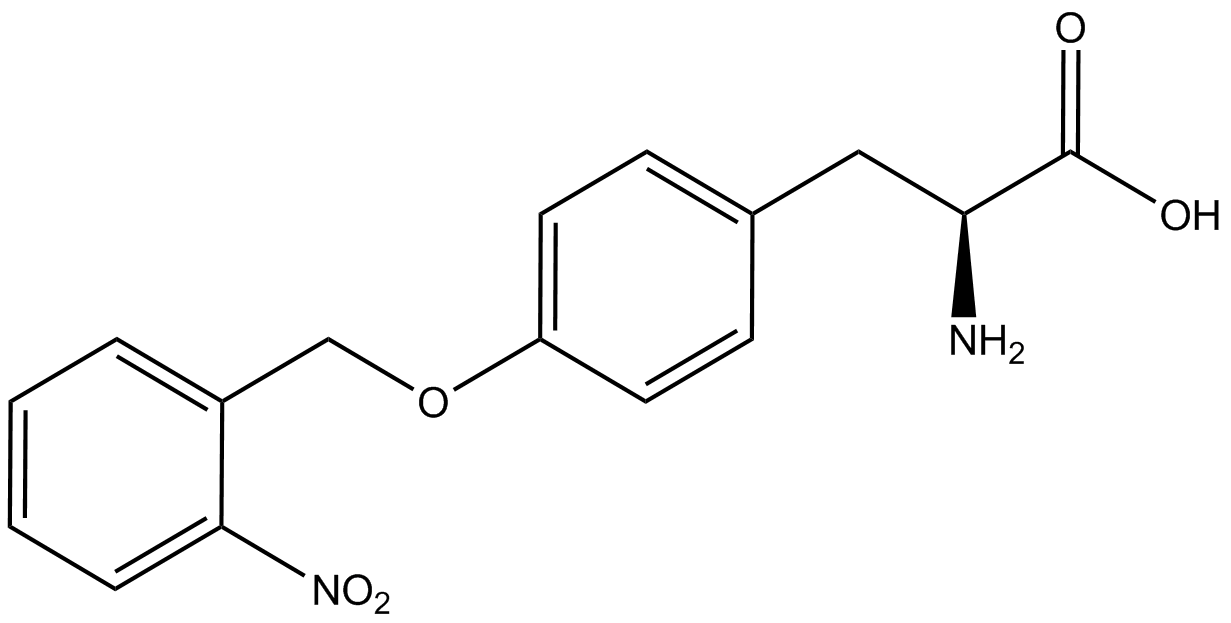
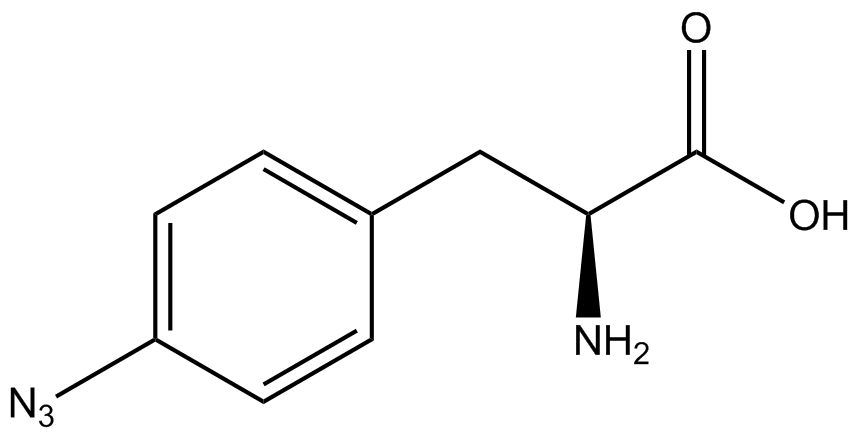
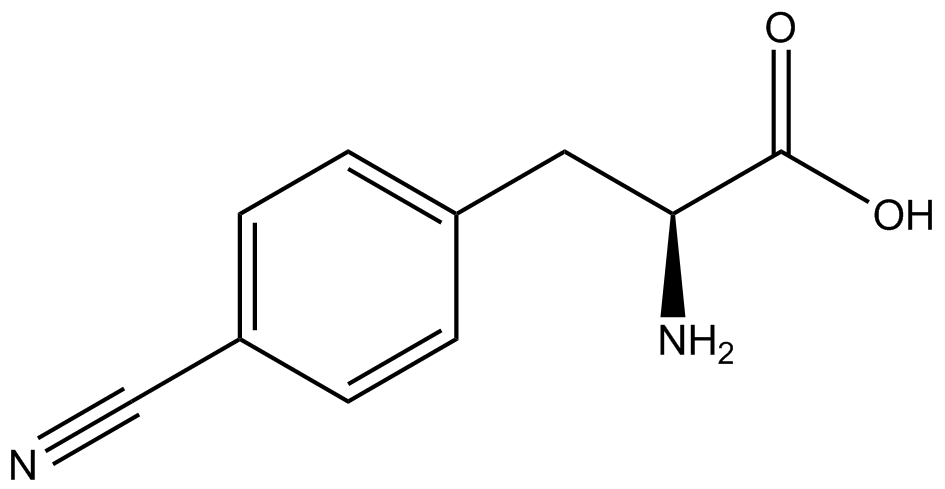
To determine the change in GFP fluorescence when the ncAA was present, we first had to calculate how much GFP was expressed relative to the RFP, which would give an upper estimate of how much GFP could theoretically be expressed. We first divided both the GFP and RFP levels by the OD 600 of the culture in order to get the per cell fluorescence levels. We then normalized the GFP fluorescence for one culture to its RFP fluorescence so that we could compare the GFP fluorescence levels between cultures. The normalized GFP values were then compared between cultures grown in the presence of ncAA and cultures grown in the absence of ncAA, which would indicate how the level of GFP fluorescence changes when the ncAA is present. When these values were graphed (Figure 3), some synthetase/tRNA pairs such as 4-azidophenylalanine, 3-nitrotyrosine, 3-iodotyrosine, and ortho-nitrobenzyltyrosine resulted in higher GFP fluorescence in the presence of ncAA than in the absence of ncAA, which suggests that those synthetases only incorporated an amino acid if their specific amino acid was present, meaning that they have a high fidelity. However, the other synthetase/tRNA pairs (3-aminotyrosine, L-DOPA, and cyanophenylalanine) did not show a significant increase in GFP fluorescence normalized to RFP fluorescence when the ncAA was present, indicating that they would incorporate other amino acids at the amber stop codon when their specific amino acid was not present (and perhaps even when it was), and thus have a low fidelity. | To determine the change in GFP fluorescence when the ncAA was present, we first had to calculate how much GFP was expressed relative to the RFP, which would give an upper estimate of how much GFP could theoretically be expressed. We first divided both the GFP and RFP levels by the OD 600 of the culture in order to get the per cell fluorescence levels. We then normalized the GFP fluorescence for one culture to its RFP fluorescence so that we could compare the GFP fluorescence levels between cultures. The normalized GFP values were then compared between cultures grown in the presence of ncAA and cultures grown in the absence of ncAA, which would indicate how the level of GFP fluorescence changes when the ncAA is present. When these values were graphed (Figure 3), some synthetase/tRNA pairs such as 4-azidophenylalanine, 3-nitrotyrosine, 3-iodotyrosine, and ortho-nitrobenzyltyrosine resulted in higher GFP fluorescence in the presence of ncAA than in the absence of ncAA, which suggests that those synthetases only incorporated an amino acid if their specific amino acid was present, meaning that they have a high fidelity. However, the other synthetase/tRNA pairs (3-aminotyrosine, L-DOPA, and cyanophenylalanine) did not show a significant increase in GFP fluorescence normalized to RFP fluorescence when the ncAA was present, indicating that they would incorporate other amino acids at the amber stop codon when their specific amino acid was not present (and perhaps even when it was), and thus have a low fidelity. | ||
| - | ==Synthetase | + | ==Synthetase Efficiency== |
| - | + | Another measure of the quality for these ncAA synthetase/tRNA pairs is how efficiently they incorporate their ncAA. An inefficient synthetase/tRNA pair will only incorporate their ncAA a fraction of the time, even when their ncAA is present. Our system can be used to measure this level of efficiency, though it does not indicate why a particular synthetase is efficient or inefficient. By comparing the level of GFP fluorescence to the normalized level of RFP fluorescence when the amino acid is present, we can see how efficient the synthetase is. In essence, if the normalized fluorescence of GFP relative to RFP is close to 100% (if the GFP is expressed roughly 100% of the time that RFP is expressed), then the synthetase is very efficient. On the other side, if say the normalized fluorescence of GFP relative to RFP is closer to 10%, then the synthetase would not be very efficient, because even when the ncAA was there, it only incorporated it at the amber stop codon (UAG) about 10% of the time. | |
| + | |||
| + | For our results (Figure 3), two synthesase/tRNA pairs stood out as relatively inefficient: 3-nitrotyrosine and ortho-nitrobenzyltyrosine. Both of these synthetase/tRNA pairs showed a significantly smaller normalized GFP to RFP fluorescence when the amino acid was present with the pFRY construct. While they both show a significant increase in normalized GFP to RFP fluorescence when the amino acid was present compared to when it was absent, which indicates a high fidelity, the actual GFP fluorescence relative to the RFP fluorescence was only around 50% for 3-nitrotyrosine and 20% for ortho-nitrobenzyltyrosine. These results suggest that for whatever reason, these synthetase/tRNA pairs do not always incorporate their ncAA at the amber stop codon. | ||
==Incorporation Value== | ==Incorporation Value== | ||
Revision as of 01:31, 17 October 2014
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"