Team:Austin Texas/kit
From 2014.igem.org
(→Discussion) |
(→Fidelity of Incorporation) |
||
| Line 182: | Line 182: | ||
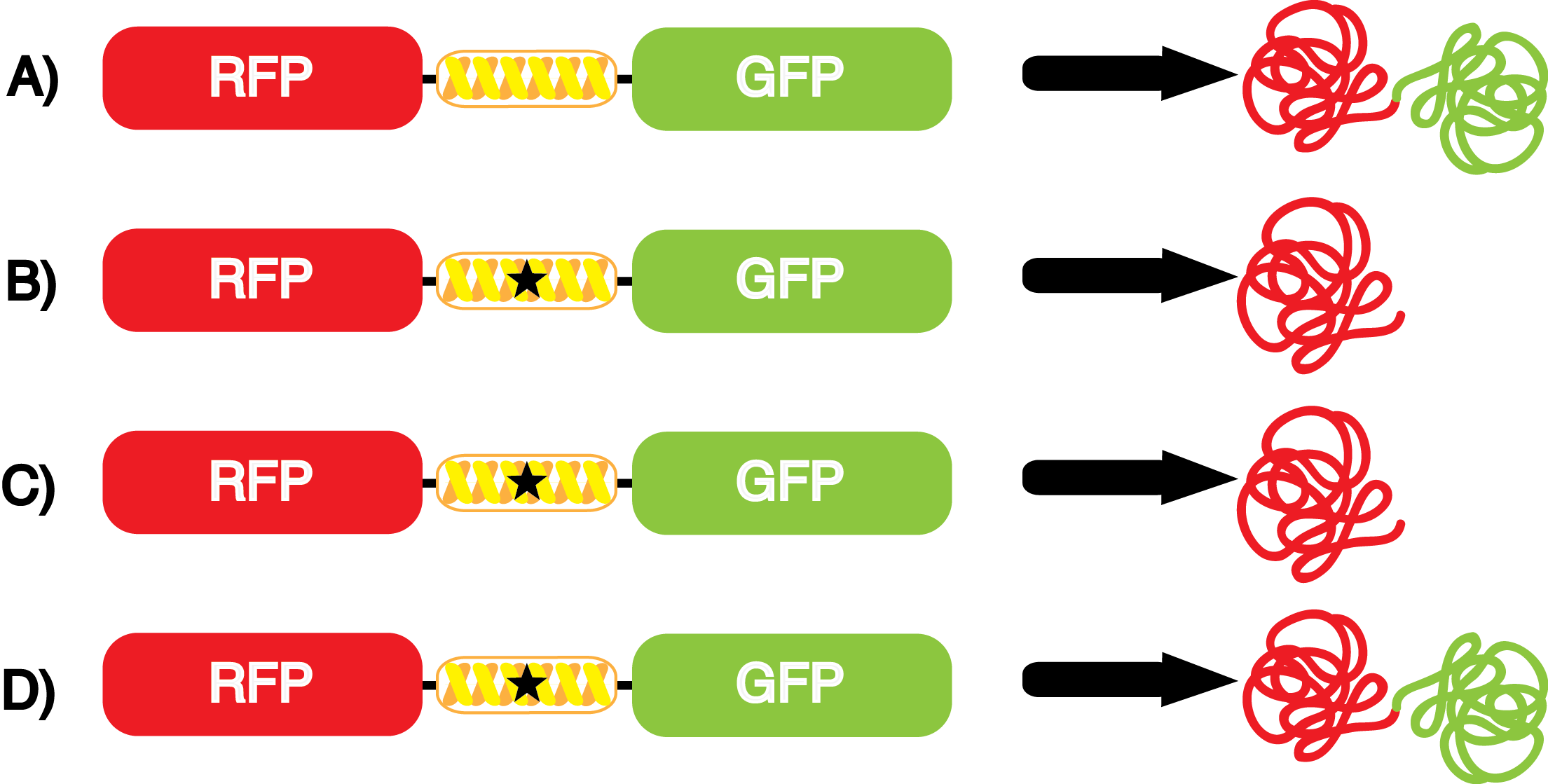
[[File:UT_Austin_2014_Kit_Normalized_GFP_to_RFP_graph.png|thumb|600px|Figure 3. Graph showing the level of GFP fluorescence relative to RFP fluorescence. Each pStG plasmid is referred to based upon the tRNA synthetase/tRNA pair present in the specific plasmid. Each of these plasmids was then paired with either pFRY or pFRYC and grown in the presence or absence of a specific ncAA. Data are presented as the average of three independent cultures. Error bars denote standard deviation.]] | [[File:UT_Austin_2014_Kit_Normalized_GFP_to_RFP_graph.png|thumb|600px|Figure 3. Graph showing the level of GFP fluorescence relative to RFP fluorescence. Each pStG plasmid is referred to based upon the tRNA synthetase/tRNA pair present in the specific plasmid. Each of these plasmids was then paired with either pFRY or pFRYC and grown in the presence or absence of a specific ncAA. Data are presented as the average of three independent cultures. Error bars denote standard deviation.]] | ||
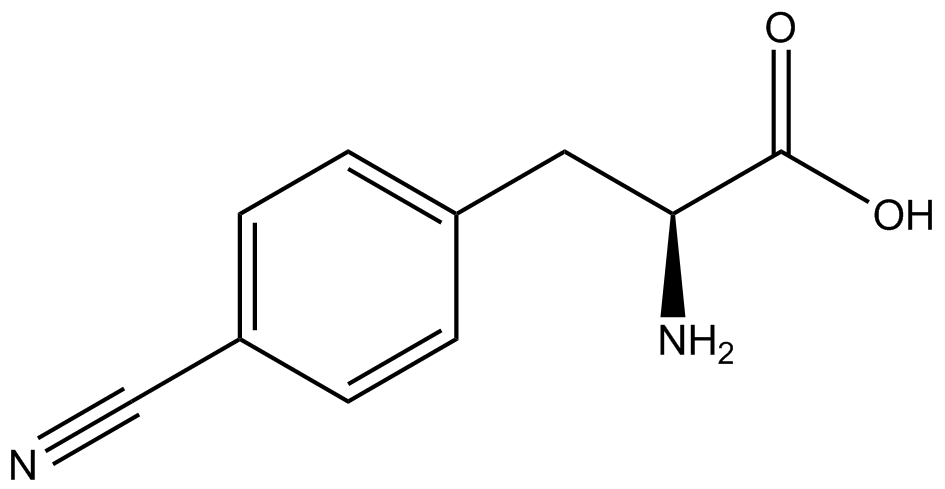
| - | The fidelity of each ncAA sythetase/tRNA pair was measured by comparing the GFP production of pStG/pFRY strains in (+/-) ncAA conditions. In the absence of a non- | + | The fidelity of each ncAA sythetase/tRNA pair was measured by comparing the GFP production of pStG/pFRY strains in (+/-) ncAA conditions. In the absence of a non-canonical only RFP should be translated, as translation is expected to terminate between RFP and GFP at the amber stop codon (UAG) on the linker sequence in pFRY. Alternatively, if the corresponding non-canonical amino acid for pStG is present or if the synthetase/tRNA pair is not specific enough, translation should continue through the linker due to its incorporation at UAG. In this case, RFP and GFP should both be translated. The amount of fluorescence from RFP and GFP is detectable by fluorometer. We also tested pStG/pFRYC strains in (+/-) ncAA conditions as a control, which should express both RFP and GFP no matter what due to the lack of the amber stop codon in the linker (with a tyrosine in its place). Without the stop codon, there would be no reason to halt translation before the GFP. |
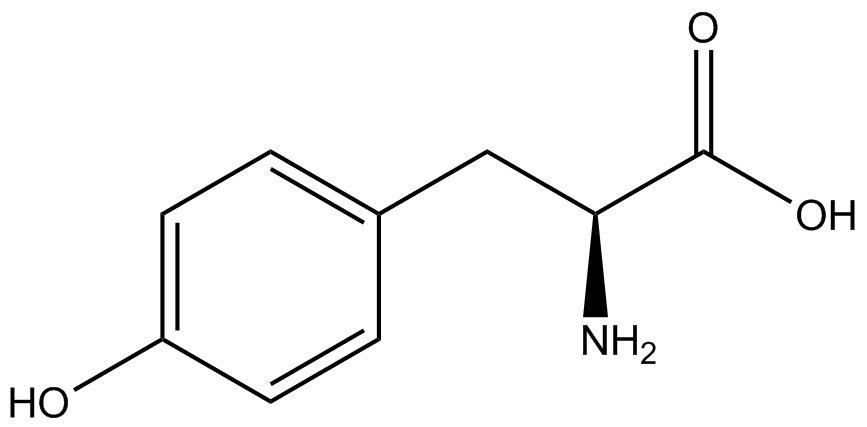
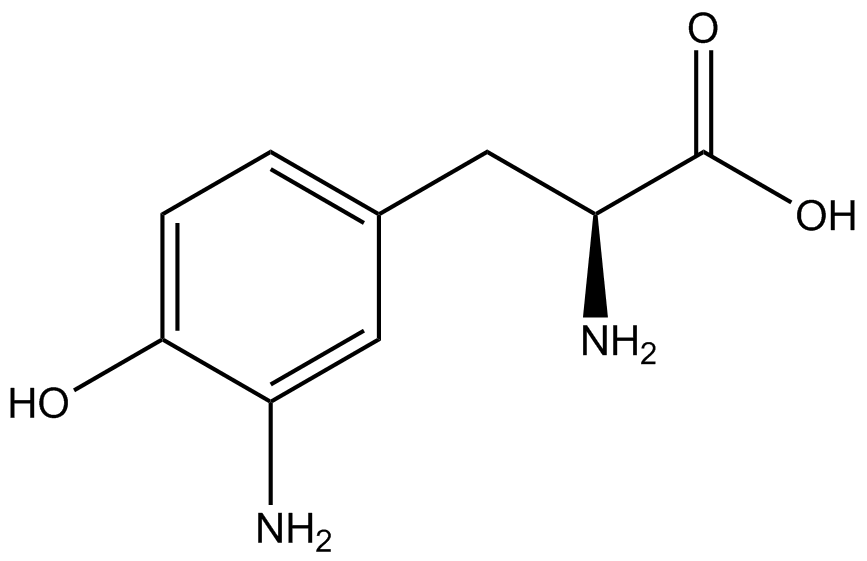
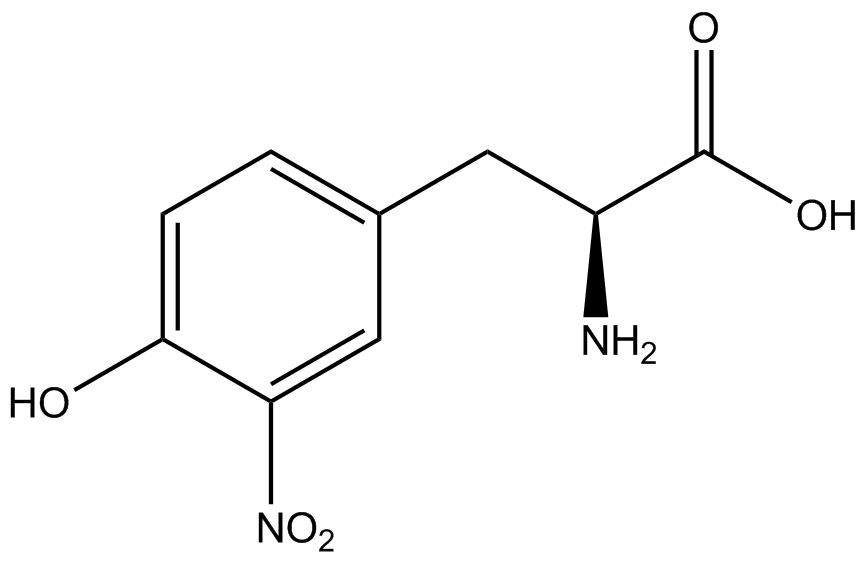
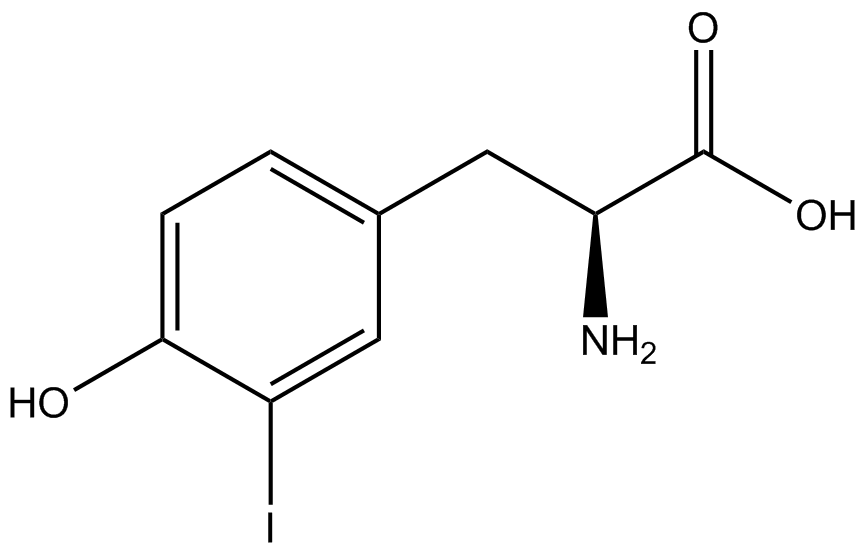
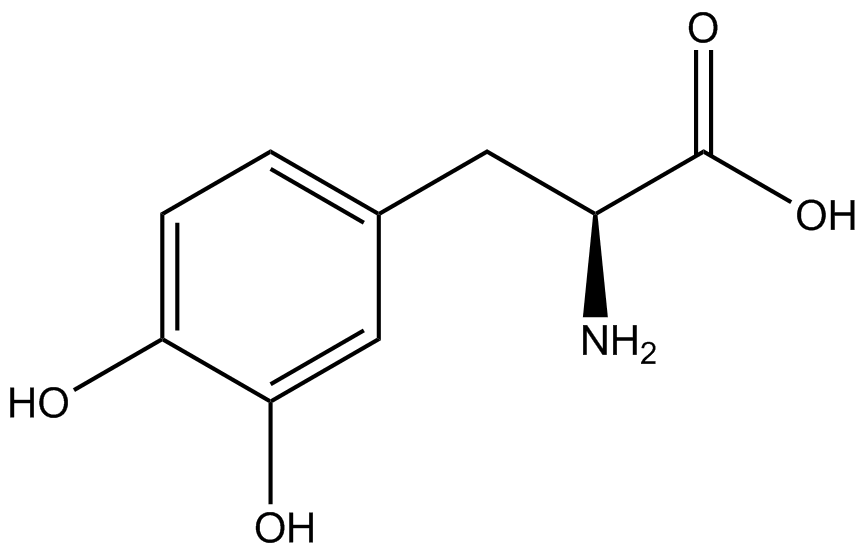
| - | Strains containing pFRY and pStG plasmids were grown in the presence and absence of the corresponding non- | + | Strains containing pFRY and pStG plasmids were grown in the presence and absence of the corresponding non-canonical amino acid. The fluorescence of RFP and GFP readings of each culture were recorded at a culture density of 0.5 OD 600. GFP values of each culture were normalized to RFP values for analysis. The normalized GFP values were compared between cultures grown in the presence of ncAA and cultures grown in the absence of ncAA. Assuming (+) ncAA cultures showed the higher GFP fluorescence, the greater difference in GFP fluorescence between (+/-) ncAA cultures, the more accurate the ncAA synthetase/tRNA pair. Most ncAA synthetase/tRNA pairs resulted in higher GFP fluorescence in the presence of ncAA than in the absence of ncAA, with the exception of 3-aminotyrosine. This suggests that 3-aminotyrosine synthetase/tRNA pair is the least accurate pair from the library tested. |
==Incorporation Value== | ==Incorporation Value== | ||
Revision as of 02:09, 15 October 2014
|
 "
"