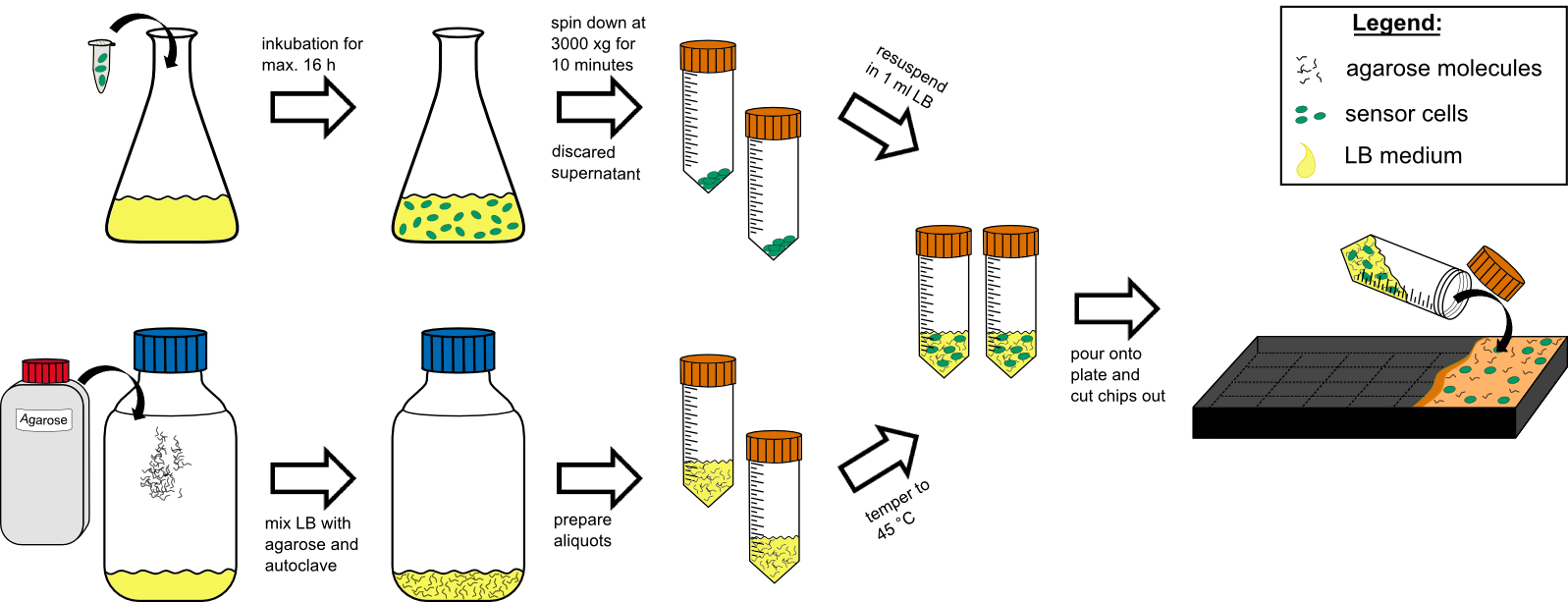
Team:Aachen/Notebook/Protocols/detection
From 2014.igem.org
(→Measurement of Fluorescence) |
(→Measurement of Fluorescence) |
||
| Line 85: | Line 85: | ||
'''Second''', we used our own device [https://2014.igem.org/Team:Aachen/Project/Measurement_Device '''WatsOn'''] with blue light for excitation (450 or 480 nm) and special filters infront of the camera for selecting the appropriate emission spectrum. You can read even more about building your own WatsOn [https://2014.igem.org/Team:Aachen/Notebook/Engineering/WatsOn here]. | '''Second''', we used our own device [https://2014.igem.org/Team:Aachen/Project/Measurement_Device '''WatsOn'''] with blue light for excitation (450 or 480 nm) and special filters infront of the camera for selecting the appropriate emission spectrum. You can read even more about building your own WatsOn [https://2014.igem.org/Team:Aachen/Notebook/Engineering/WatsOn here]. | ||
| - | '''Third''', we used the | + | '''Third''', we used the '''Synergy Mx microplate reader''' (BioTek), putting the sensor chips into the lid of a common clear well plate. GFP was measured with an excitation of 496 ± 9 nm and an emission of 516 ± 9 nm and iLOV with an excitation of 450 ± 9 nm and emission of 495 ± 9 nm. |
<html> | <html> | ||
Revision as of 01:25, 17 October 2014
|
 "
"