Team:UChicago/Notebook
From 2014.igem.org
You should make use of the calendar feature on the wiki and start a lab notebook. This may be looked at by the judges to see how your work progressed throughout the summer. It is a very useful organizational tool as well.
This needs to get split up into separate pages somehow
Contents |
Week 1 6/16 - 6/20
Monday
First day of lab! We plated some DH5-alpha competent cells in preparation for gDNA extraction for our PCRs and future transformations. One person showed up (Kevin yayyyyy), then left.
- picture of Kevin here*
Tuesday
We attempted to make LB plates. We had fun watching it boiling over since we left it in the hot plate too long and it spilled everywhere. We did clean-up time afterwards! In the future, we will remember to to heat for only 1 min to dissolve everything except agar. Agar will dissolve during autoclave because science is cool. So exciting! :D We then remade the LB for funsies :D We did a few other things to start things up like making SOB, CCMB80 competent cell buffer, and antibiotic plates.
Wednesday
MOAR BUFFERS! Qiagen buffers are expensive so we spent the day making bootleg Qiagen miniprep buffers (sorry Qiagen, you had your chance). Then, we contemplated life for the rest of the day. *take depressing pictures here*
- take pictures of buffers*
Thursday
Made competent cells. Stuck hands in liquid nitrogen. Burnt our hands off (no pics because you need hands to use a camera, duh). EVEN MOAR BUFFERS We also went to a biotech company exhibit and grabbed a lot of free samples! (and food of course) We also extracted gDNA from DH5a to PCR out our mutator genes. We also learned not to use the free 1.5mL centrifuge tubes from last year, and to not smell our microcentrifuge anymore. Stock strains MG1655 and delta-tyrR came in from the Yale E Coli Stock Center. Yay! So exciting! Kevin was so excited that he burnt his hair trying to plate them. XD
We made competent cells (MY CRYSTAL BALL SAYS: THEY WILL FAIL). Kevin BS’d the gDNA and got some somehow but also made the centrifuge stink like chloroform/phenol. Fun XD
This company (name concealed) gave us some cool centrifuge tubes last year that warp when we spin them down. XD So we’re buying new ones. $$$ They’re wrong- money DOES grows on trees! AND we have lots of trees around here! Even better! We ran overnight PCRs, one with more and one with less cycles.
Friday
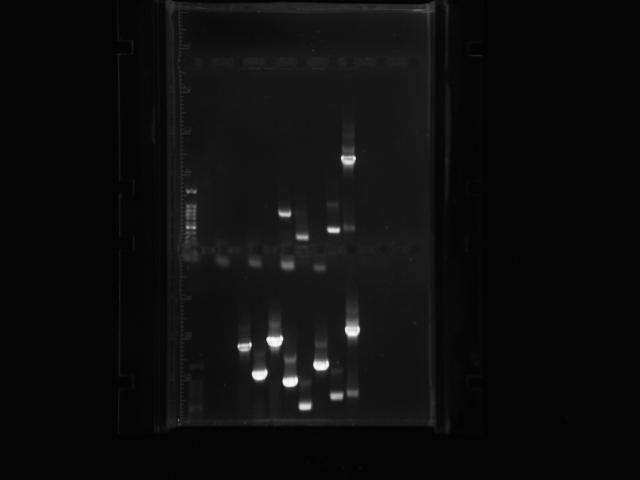
PCR-ed out 9/12 mutators. The lengths of almost all of them were verified to be correct!!! So much happy!!! Yes!!!! Yay!!!! The PCR with less cycles (bottom half of the gel) worked better so we’ll stick with that in the future. We also digested backbones that have the RBS we will use and ligate to all the mutators. What a good day!
Week 2 6/23 - 6/27
Week 2 summary
Monday
We tested our competent cells using the transformation efficiency kit. They didn’t work XD But that’s okay, we like making competent cells. It’s strangely exciting to pipette things lots of times :D
Also, we fragment isolated the mutations and then did the restriction digests on the PCR products. The concentrations of the genes are fairly alright, about 20-50 ng/microL. We ran a gel of AroF to verify PCR of the gene, but unfortunately we did not get a band XD. That’s okay, life goes on even if your gels fail!!! XD IT’S summer time! Rainbows!
We gel extracted the mutators that were digested last week. Fun. Got high contamination. But contamination is good because what doesn’t kill your experiments makes them stronger right ? Yes! Concentrations are listed below XD
| !Sample | !Conc (ng/microL) | !260/280 | !260/230 |
| ParC | 30.2 | 1.78 | 0.25 |
| Butter | Ice cream |
Sample Conc (ng/microL) 260/280 260/230 1:ParC 30.2 1.78 0.25 2:DinB 55.8 1.70 0.42 3:MutS 21.3 1.86 0.18 4:Dam 37.0 1.68 0.18 5:MutT 29.9 1.61 0.09 6:Umu’C 24.5 1.80 0.05 7:EmrR 36.8 1.71 0.08 8:DnaE 31.1 1.53 0.32
Tuesday
NOTHING WORKED :)))))!!!!!!!! We drank our worries away XD XD XD. We drunkenly made glycerol stocks of the two strains from Yale that came last Thursday.
- hic* guuysh guysh we should *burrrrp* totally ligate a rBs to a vecter *hi*c
Alcohol is poison. (Delicious, delicious poison. And we are Mithridates.)
Unfortunately, our cells weren’t competent when we tested them again :( We went over the protocol and made sure we’ll have everything down for next time XD. Or rather, fortunately! We get to make more! XD Soooo stoked! We fragment isolated and gel purified our promoter plasmids. For our project planning, we met with our faculty advisors Mike and Jennifer. The meeting was super, super, super productive and educational! We could consider inserting a polylinker into our plasmids to reduce a lot of steps XD Polylinkers are fun!
Wednesday
We purified the 8 mutators that we PCR-ed out. As for AroF and the three mutators that didn’t work, we tried PCR with Phusion, but unfortunately it didn’t work again. Yay! After digesting our RBS and ligating it with our previous cut promoter plasmids, we also did 3A assembly in parallel for RBS and promoter. Yay! More amp and tet plates were made! Yayyy!
- picture of plates”
Thursday
We had issues with SOC contamination today - We had to make a new batch for more transformations. We also tried transformation with the efficiency kit again, making sure we were following each step as it was written on our protocol.
Friday
Transformation of our 3A assembly colonies didn’t work, but the transformation of mcherry plasmids worked! This means that there’s nothing wrong with our transformation protocol. We will look into our digestion and ligation steps. We <3 troubleshooting! All part of the scientific process XD!
Here’s a joke XD:
What do you call it when McDonald sells cherries?
mCherry! HAHAHA XD!
We made more chloramphenicol plates again! Yay!
 "
"