Team:SUSTC-Shenzhen/Project/Cell Line Construction
From 2014.igem.org
Cell Line Construction
Brief summary of cell line construction!
Contents |
Introduction
The fancy idea to equip human cells with an intracellular self-protecting immune system against lentivirus especially HIV is about to realize by introducing Cas9 protein into the genome. However, does Cas9 protein have sufficient efficiency to disrupt targeted virus DNA sequence and disable it to free patient from lentivirus caused disease. Does the side effect acceptable. These still remain unknown questions, need more experiments to determine. Our strategy to cure and prevent AIDS is to integrate Cas9 gene into hematopoietic stem cells. When HIV infects human, plasmid encoding appropriate gRNA sequence targeting HIV sequence will be introduced into the transformed stem cells, then HIV genome will be cut off by Cas9 protein.
Experiment Design
Will it works? To verify we design 3 steps of experiments.
The first step:
We use HeLa cells and EGFP to simplify the experiment because using hematopoietic stem cells and HIV is impractical for us. We will test the idea in HeLa cells which is a common used immortal human cell line (wikipedia) and the EGFP gene will be a substitute of HIV gene firstly. Both Cas9 gene and EGFP gene are stably transfected into HeLa cells, which mimics that the hematopoietic stem cell equipped with Cas9 are infected by HIV. Then the plasmid encoding gRNA targeting EGFP gene is introduced into the cells to measure the efficiency of the Cas9 protein to cut off the targeted sequence with the guide of gRNA.
The second step:
Whether the targeted sequences of HIV screened out by our team can be efficiently disrupted and safety of our target sequence will be tested. To make the result easier to detect and measure quantitatively, the virus sequences will be inserted into EGFP gene by locating the sequence before or on the both ends of the EGFP gene. Then the plasmid encoding gRNA to target virus sequence will be introduced into the cells to see whether the virus sequence can be disrupted or eradicated successfully.
The third step:
The third part is A-B toxin delivery, to respond to the high mutation rate of HIV. In other words, different gRNAs will be delivered into the helper T cells of the patient whose hematopoietic stem cells have been equipped with Cas9 gene. A safety In vitro delivery of DNA into targeted cells specifically is needed. In our project, two fusion proteins TEG and GD5 which contain a domain mimics bacteria A-B toxin are used for DNA delivery.
The construction of HeLa cell line stably transfected with EGFP gene and Cas9 gene.
To obtain a cell line with EGFP gene and Cas9 gene integrated in, we used PiggyBac transposon system to stably transfect. Then expand mono-clones and population expressing resistance into cell line.
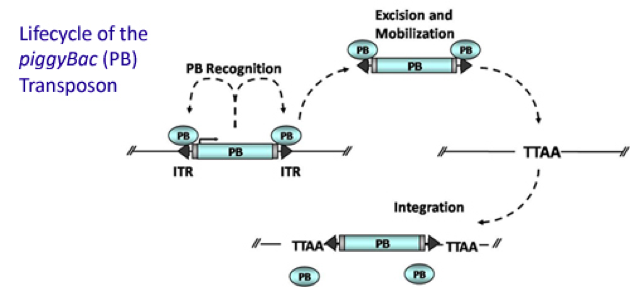
Figure 1. Mechanism of PiggyBac transposon system
It is estimated that only part of the transfected cells will be integrated with Cas9 gene and EGFP gene. Resistance screening we have used to get a pure cell pool of transfected cells. Plasmid Cas9-TetOn expressing Cas9 under the control of Tet-ON 3G using Piggybac transposon system encodes puromycin resistance, and plasmid PB-EGFP to stably express EGFP in host cells using Piggybac transposon system encodes blasticidin resistance.
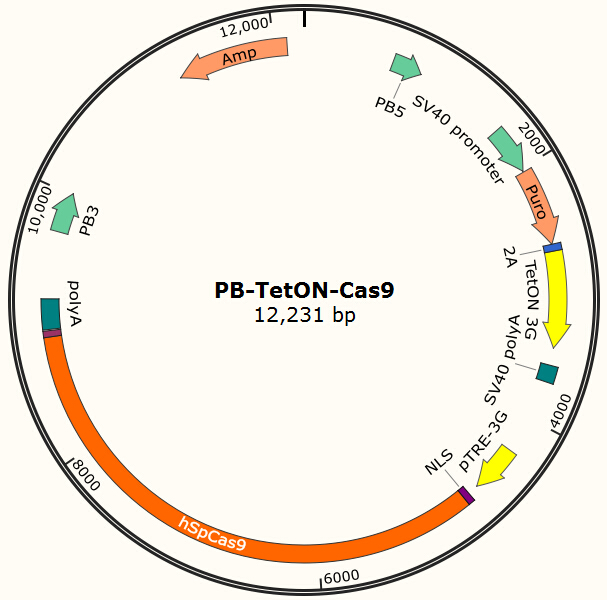
Figure 2. PB-TetON-Cas9 plasmid, PB5-PB3 are transposon, Puro is resistance gene.
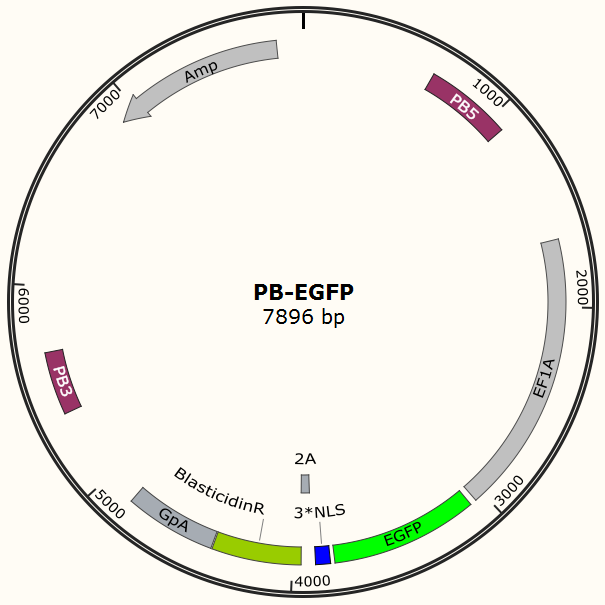
Figure 3. PB-EGFP plasmid, PB5-PB3 are transposon, Bla is resistance gene.
To determine the minimum usage level of puromycin and blasticidin in culture medium to inhibit the growth of normal HeLa cell, we culture cells in medium with gradient concentration in different wells of cell culture plate. We found that the minimum efficient concentration of bla(blasticidin) is 2.8μg/mL (More Details)and puro(puromycin) is 2.0μg/mL.(More Details)
After about one month selection, the percentage of HeLa cells expressing EGFP was about 95% (Figure 4 & 5).
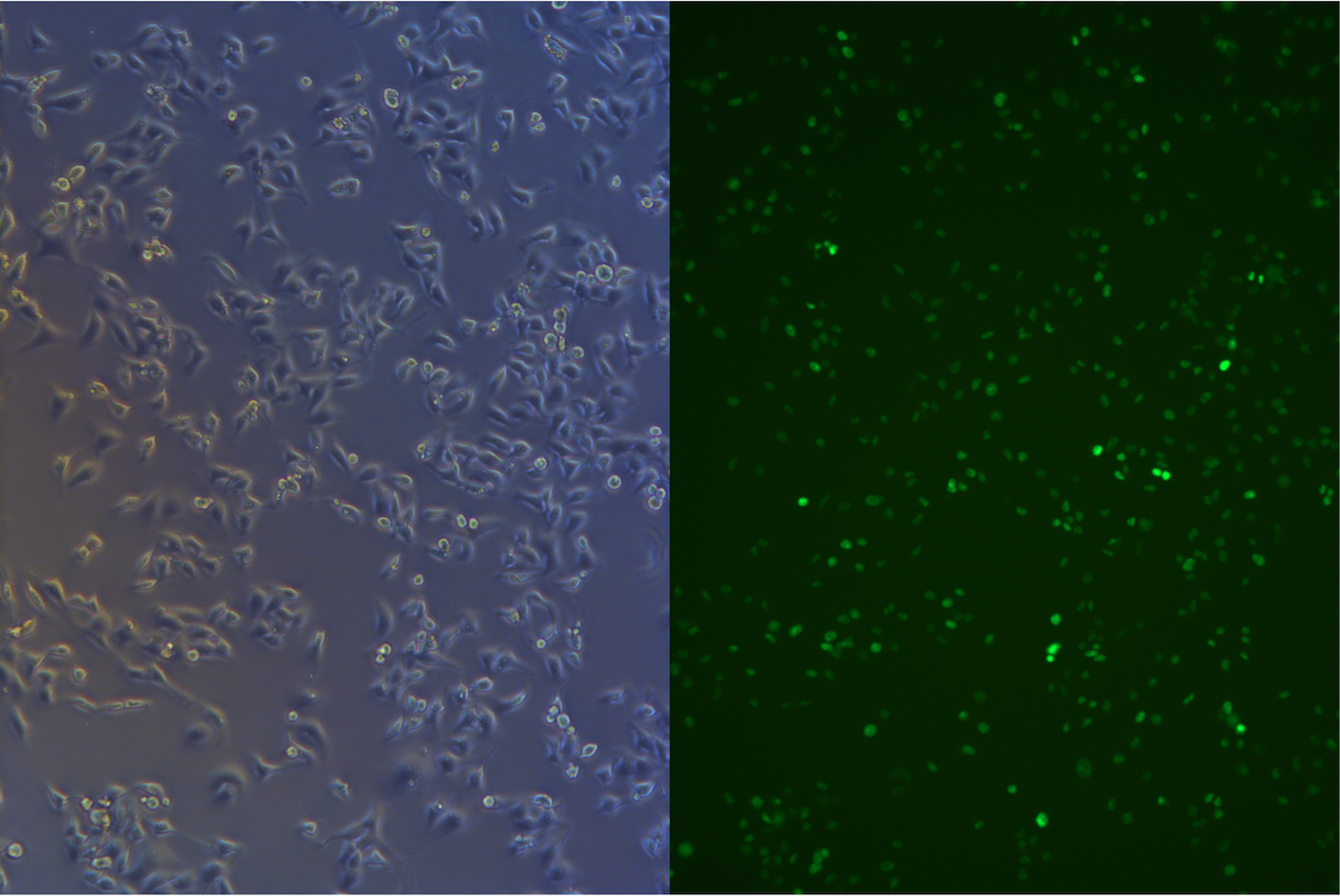
Figure 4. Pool cells transfected with EGFP gene before freezing.
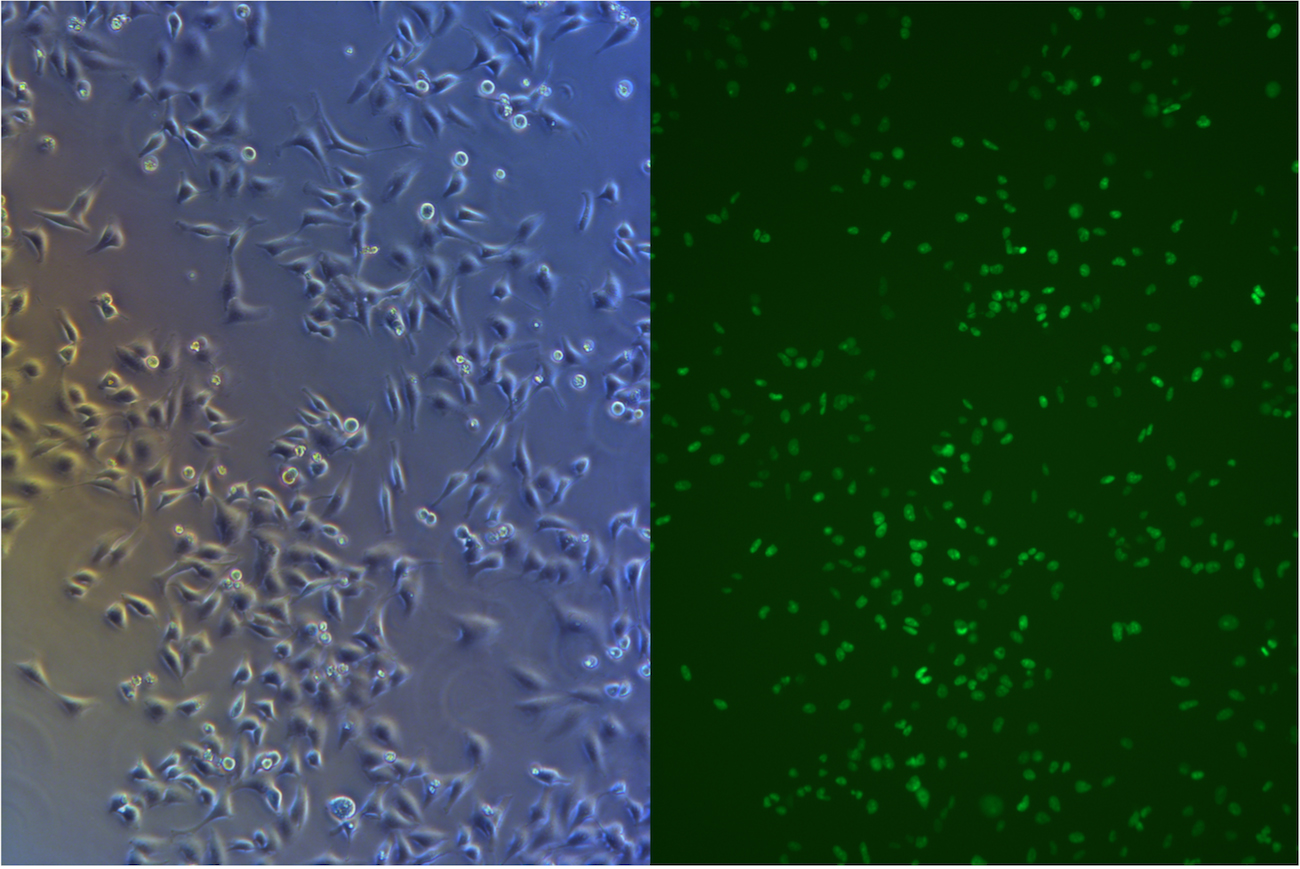
Figure 5. Pool cells transfected with EGFP gene and Cas9 gene before freezing
Monoclonal cells are supposed to have the same insertion sites and copy number which make the system start with fewer variables. Moreover, monoclonal cells with low copy number of EGFP gene is preferred and constant copy number of Cas9 gene. Cells with high copies of EGFP gene may conceal the damage of part of them caused by Cas9 and therefore underestimate the efficiency of Cas9 protein and gRNA.
So we have also cultured some monoclonal cells whose fluorescence intensity appeared to be lower. Comparing with the pool of the cells stably transfected with EGFP gene and Cas9 gene, the monoclonal cells has a smaller EGFP intensity range. Then the several monoclonal cells are transfected with plasmid encoding the gRNA for EGFP. On the whole, the percentage of the cells with EGFP signal in all clones transfected with plasmid encoding gRNA decline dramatically, decline from 91%- 99% to 50%-79%(Table 1, Figure6). (More Details)
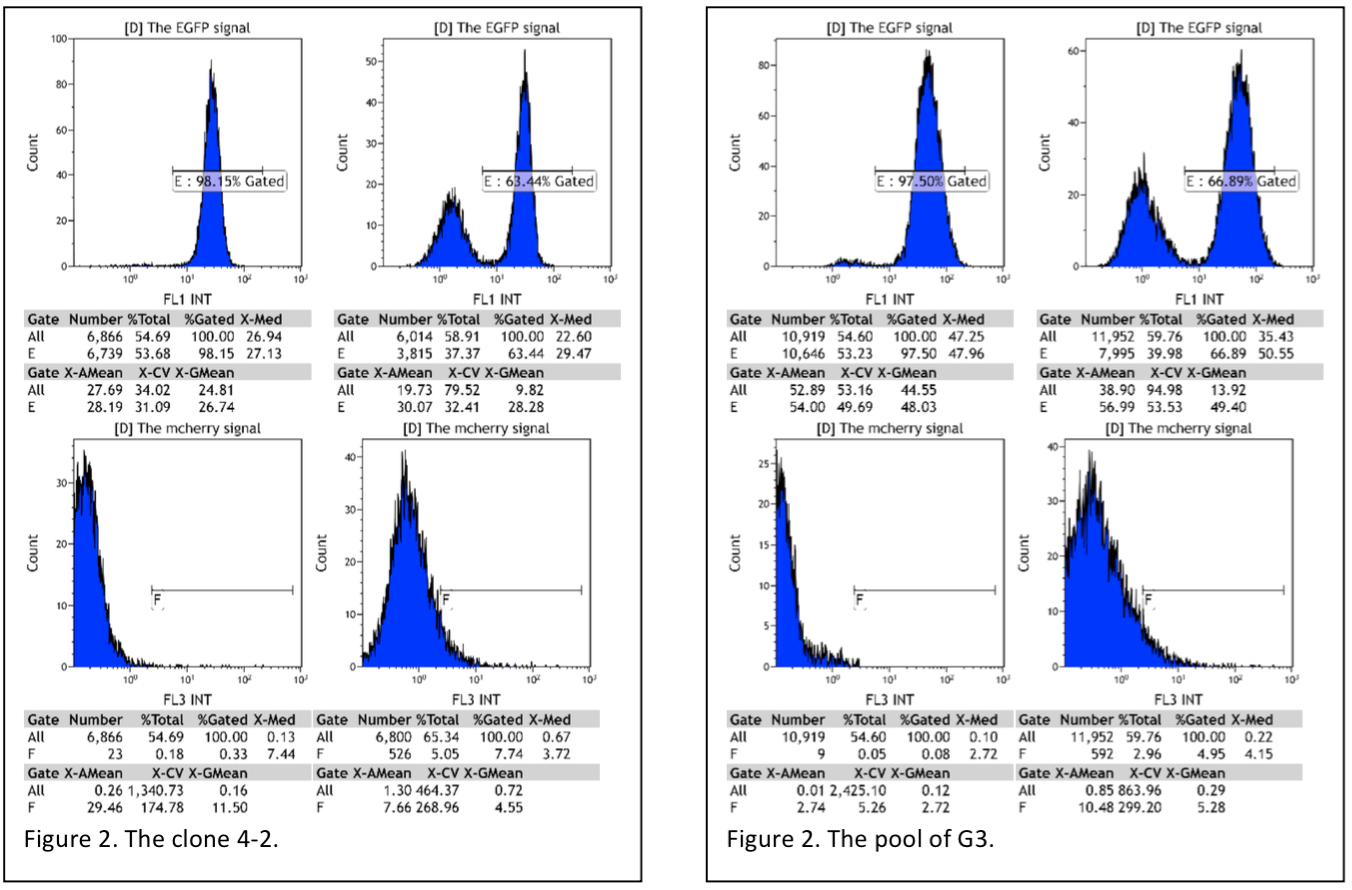
Figure 6. Flow cytometry reading pool cells(G3) and monoclones(4-2).
| Clones | The change of the percentage EGFP signal | The change of the mean of the intensity of the EGFP signal of the positive cells |
|---|---|---|
| A1 | 91% to 79% | 34 to 40 |
| A2 | 91% to 79% | 35 to 18 |
| B5 | 97% to 57% | 33 to 19 |
| C5 | 99% to 67% | 34 to 17 |
| 4-2 | 98% to 64% | 28 to 20 |
| G3 pool | 98% to 67% | 53 to 39 |
Table 1. The summary of the data of flow cytometry, reading monoclones integrated with Cas9 and EGFP gene.
 "
"