Team:Heidelberg/Project
From 2014.igem.org



iGEM TEAM HEIDELBERG 2014
THE RING
OF FIRE
Click here to view our abstract.
Scroll down to EXPLORE our project.
CIRCULAR PROTEINS
Nature has made many curious inventions. One of these are circular proteins, which are conventional peptides that neither have a beginning, nor an ending
Apart from other features, these proteins are extremely stable against high temperatures, pH and proteases.
We seeked to apply this principle of circularization in Synthetic Biology and create a way of rendering any protein heatstable.
Let us take you to the next level of bioengineering ...
USING THE Mechanism OF SPLIT-INTEINS
Intein splicing is a natural process that excises one part of a protein and leaves the remaining parts irreversibly attached.
When attaching split inteins to the ends of a normal protein, the splicing reaction connects the beginning to the ending and forms a circular protein.
... and show you the WORLD of
post-translational MODIFCATION
The iGEM Team Heidelberg has developed an intein toolbox for the iGEM community to easily modify your protein in a standarized method.
Our toolbox contains several tools which are PLACEHOLDER. Here you can find out more about our Toolbox.
In addition to that all tools are inducible by light. Using the LOV system we built a solid method for regulation of the intein trans-splicing reaction our toolbox consiting on. Click here to get more informations about Induction.
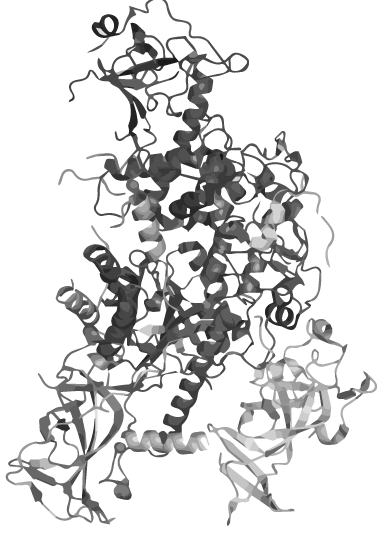
circular heat-stable
DNMT1
Wouldn´t it be great to amplify DNA in a normal PCR maintaining the epigenetic information coded in methylation patterns?
The problem: DNMT 1, an enzyme which is responsible for the establishment and maintenance of the individual methylation pattern of different cell types, is not heat stable. For iGEM 2014 we therefore create a PCR 2.0 with heat-stable DNMT 1 by circularization.

circular heat-stable
Xylanase
Xylanase is an important enzyme for the pulp and paper industry.
Bla bla
In future Xylanase could be used for the production of biofuel.
LINK it!
Could every protein becomes heat stable by circularization, even if it´s the most complex of all?
Circularization is a narrow path between gaining heat-stability and loosing function due to deformation. We developed a linker software, which predict the perfect linker depending on the folding structure of every protein.
In an extensive linker screening our software was improved and calibrated using the lambda phage lysozyme.

CALCULATE it!
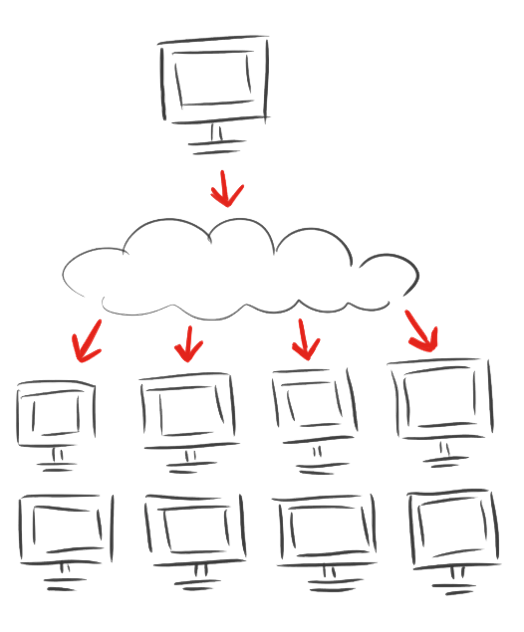
After calculating for eleven days and the breakdown of both computational and mental power we decided to spread the modeling of the linkers.
The iGEM Team Heidelberg developed iGEM@home, a software to divide extensive computing task into many packages and to distribute them to many computers. Now over 1,000 volunteers are calculating for us when their computers are idle.
As a new tool for the iGEM community this system enables every student team to archieve their modeling without access to big server farms.
Who are we?
We are the iGEM Team Heidelberg 2014 consisting of 12 highly motivated bachelor and master students studying at Heidelberg University.
For our project we got great feedback and support from our supervisors.
Take a look at our Teampage!
Thank you!
We want thank all people who helped us and supported our work in the lab.
 "
"
 CIRCULARIZATION
CIRCULARIZATION
 OLIGOMERIZATION
OLIGOMERIZATION
 FUSION
FUSION
 ON/OFF
ON/OFF
 PURIFICATION
PURIFICATION