Team:NCTU Formosa/project
From 2014.igem.org
- Overview
- PBAN
- Biobrick
-
Overview
Insect damage has been a serious problem for a long time over the whole world and it is difficult to be solved. People have took several methods to kill these harmful insects, but the methods have caused other problems to the environment. In order to solve these troublesome problems and not to cause side effect, we established an alternative way to solve it in an eco-friendly manner.
-
PBAN
PBAN (Pheromone Biosynthesis Activating Neuropeptide) is a simple, short peptide. When it binds with a receptor on the pheromone gland of an insect, it will activates pheromone synthesis. Just like the pheromones, PBAN is species-specific, so we can use one kind of PBAN to enhance pheromone biosynthesis on the kind of insect that we are targeting.
-
Brobrick
In our basic design, we want to express PBAN in an easier way than the original complex process. To make it more convenient to observe and model, we ligate the reporter gene BFP into our biobrick.
Contents
|
Overview
Impact of pest
From ancient times to the present, harmful insects always play roles in our life, and we don’t have any appropriate solution to these annoying pests. Every year, harmful insects cause a great amount of loss to our agriculture, which seriously disturbs our people livelihood and economy.
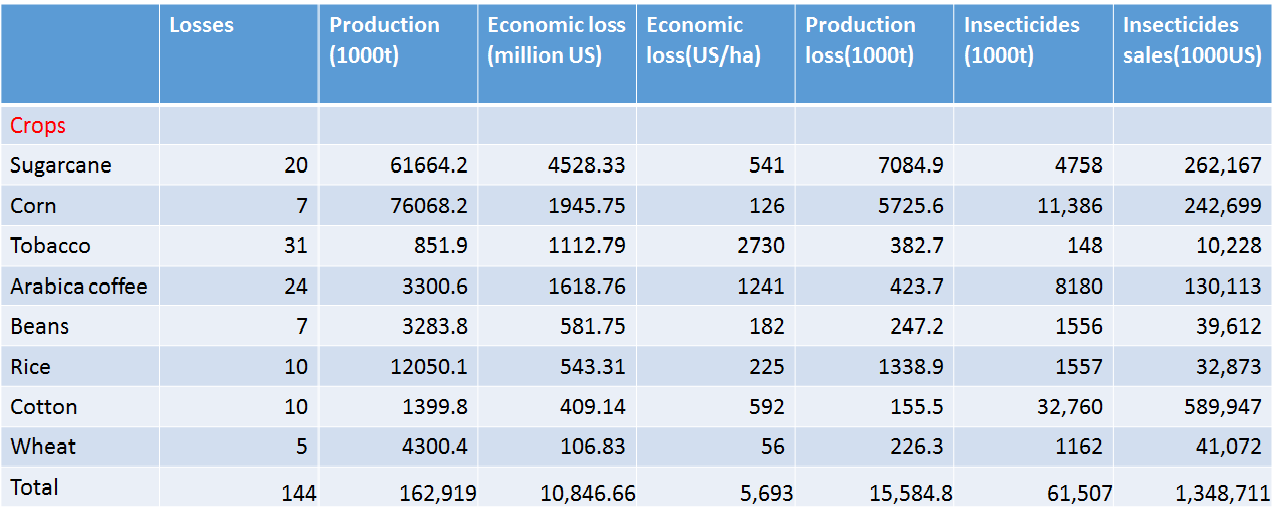
In order to make our discuss more trustworthy, we found some data to support our opinion. Firstly, we take Brazil for example. We can find several crucial economic crops such as coconut, coffee, and sugarcane are damaged by harmful insects. The total loss of crops in Brazil is about 11 billion US dollars. In addition, we can also find that people spent nearly 1.4 billion controlling these annoying pests. What's more? They have used a great number of insecticides containing some toxic substances. However, this method will make human beings exposed to horrible situation.
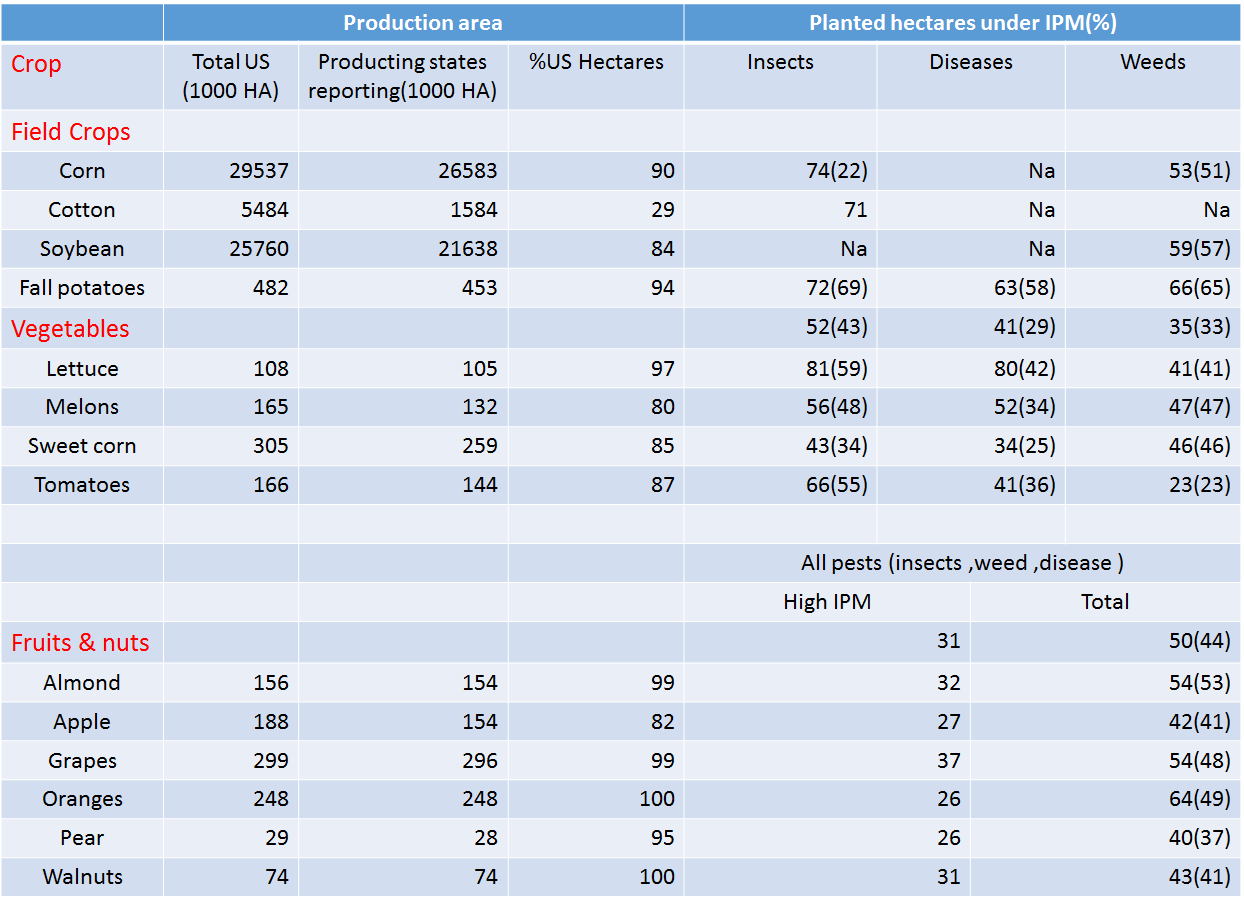
losses caused by pests in BrazilSecondly, there is also a data about the IPM (Integrated pest management) of USA. IPM means the ability to control pests. The IPM is reality number in global, and the lower of the number is, the more efficiently we control. In the data, we can see that lots of areas in USA are used to plant crops. There are several kinds of plants which are still under attack by harmful insects. We can only control the insect damage fairly well in a small limited production area. Therefore, as the production areas expand to an extent, the effect of control declines to a point. Even though the production areas become larger, the total production is lower. Therefore, what we need is invent a brand new method to solve this problem.
chemical control method
Because of the hazard of insects, human beings have come up with lots of ideas to kill these harmful insects. In the 15th century, people used heavy metals such as Arsenic, Mercury and Plumbum to kill harmful insects, which caused a catastrophe to the environment. Pesticides became more powerful along with the technology, in the 20th century, the agriculture developed rapidly just because of the evolution of pesticides. But the pesticides are not only fatal to the insects but also harmful to the human beings. People found this problem after several decades. The toxin of the pesticides will be kept in creatures by the food chain, and finally, goes into human's body. However, it's not too late to improve this situation. We can create an evolution of agriculture by a new method, and that is what we do!
Damage to human body
Pesticides includes substances that kill weeds (herbicides), insects (insecticides), fungus (fungicides), rodents (rodenticides), and others. The use of toxic pesticides to manage pest problems has become a common practice around the world. Pesticides are used almost everywhere, causing human health hazards, ranging from short-term impacts such as headaches and nausea to chronic impacts like cancer and reproductive harm. There is also mounting evidence that exposure to pesticides disrupts the endocrine system, wreaking havoc with the complex regulation of hormones, the reproductive system, and embryonic development.
Damage to the environment
Pesticides wreak havoc on the environment, threatening biodiversity and weakening the natural systems upon which human survival depends. Pesticides have led to abnormal mass mortality of America's honeybees. The populations of bees have dropped by 29% to 36% each year since 2006. To our astonishment, fully 1/3 of the food we eat depends on bees for pollination!
Secondly, some scientists believe that amphibians and bats have become more susceptible to deadly disease because their immune systems are weakened by pesticides. What's more, one kind of pesticides called herbicide, which can give a male frog a sex change. Genetically, the frogs are still males, but morphologically they are completely female and they can even mate successfully with other males and lay viable eggs. Pesticides have also contaminated waterways and endangered fish and birds, causing ecological damage.
By the way, pesticide resistance makes insecticides ineffective. Other factors in the speed with which a species evolves resistance are generation time and fecundity, that is, causing shorter generations and more offspring lead to resistance more quickly.
Biological control method
Since most chemical control methods are poisonous to the environment, humans began to look for other alternative ways. One of the most well known example is bacillus thuringiensis (Bt), which can efficiently kill insects with its crystal proteins. However, recent researches have shown that more and more insects have been resistant to this kind of protein. It's time to find other better solutions.
Solution to both questions
People invented pesticides to eliminate the pests, but the use of pesticides hurt the environment and cause harm to humans. Thus there have been conflicting issues, and have remained to be settled. In order to solve the above-mentioned problems, we came up with a practical, inexpensive way.
Our target insect
Check photo to see introduce

|
 |
 |
|---|---|---|
 |
 |
 |
 |
 |
 |
Reference
-
1.Pogue, Michael. "A review of selected species of Lymantria Huber [1819]". Forest Health Technology Enterprise Team. Retrieved September 14, 2012.
2.Eliminate Cutworms Using Natural Pest Control By Susan Glaese March/April 1987
3. Laurence Mousson, Catherine Dauga, Thomas Garrigues, Francis Schaffner, Marie Vazeille & Anna-Bella Failloux (August 2005). "Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations". Genetics Research
4. NPIC is a cooperative agreement between Oregon State University
5.M. S. Ascunce et al., “Global Invasion History of the Fire Ant Solenopsis invicta”, Science, vol. 331, no. 6020, pp. 1066 - 1068, 2011
6.organicgardening.com/learn-and-grow/fire-ant-control
7.Scientists suggest fighting fire ants with ice By Chiu Yu-Tzu / STAFF REPORTER
8.Capinera, J.L. 1999. Fall armyworm Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae
9.Helicoverpa Diapause Induction and Emergence Tool Introduction to the Helicoverpa armigera Genome Project Helicoverpa armigera Genome Project updates on InsectaCentral Helicoverpa Genome Project database on-line Lepiforum Funet Taxonomy Fauna Europaea
UC IPM Pest Management Guidelines: Cotton UC ANR Publication 3444
10. "Interesting (To Us) Photos From The Garden". Meades.org. Retrieved 2011-08-11.RXwildlife Sightings » Blog Archive » More Emergence". Rxwildlife.org.uk. 2009-06-03. Retrieved 2011-08-11.Cabbage Moth - Caterpillar". Habitas.org.uk. Retrieved 2014-08-11.
11.Authored by W. H. Reissig. Published by the New York State Agricultural Experiment Station, Geneva, A Division of the New York State College of Agriculture and Life Sciences, A Statutory College of the State University, Cornell University, Ithaca. Funded in part by an Extension Service-USDA, IPM Grant.
PBAN(Pheromone Biosynthesis Activating Neuropeptide)
Introduction of Pheromones
A pheromone is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting outside the body of the secreting individual to impact the behavior of the receiving individual.
There are many kinds of pheromones such as alarm pheromones, food trail pheromones, sex pheromones, and many others that affect behavior or physiology.
Our project - Operation Debug aims to use different PBAN from different moths and other insects to induce them to make their own sex pheromone.
Sex pheromones, which are an important factor in finding a mating partner. When a female releases chemicals, the mate search is initiated, and the male moths begin their upwind motion toward their potential partner. Sex pheromones in particular are associated with long-range chemical communication of sex substances used in signaling a mating partner. Mate finding in moths involve sex pheromones that have the ability to propel long-distances and are emitted by the females abdominal glands in most cases.
Ways to make pheromones
There are 2 main ways to make pheromones. One is chemical synthesis, the other one is biosynthesis approach.
1. Chemical synthesis approach:
It is difficult to make pheromone by chemical synthesis approach. This approach needs to use many kinds of chemical compound which may cause some pollution to the environment. Furthermore, chemical approach needs to control the condition of reaction that may consume a lot of energy. An example of chemical synthesis approach: leafroller moth, Bonagota cranaodes.
2.Biosynthesis approach:
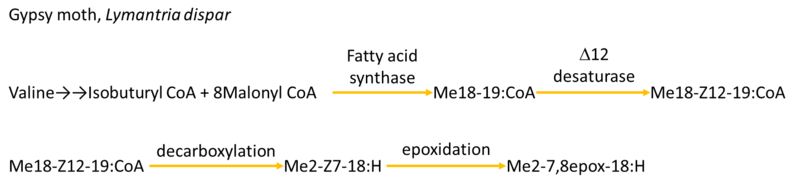
Biosynthesis approach needs lots of enzymes to catalyzed, which is too complicated for E.coli to carry out. In addition, E.coli is lack of Glycoproteins that can do posttranslational modification. Therefore, it is nearly impossible for E.coli to produce pheromones in a normal way. An example of Biosynthesis approach: Gypsy moth, Lymantria dispar.
Introduction of PBAN
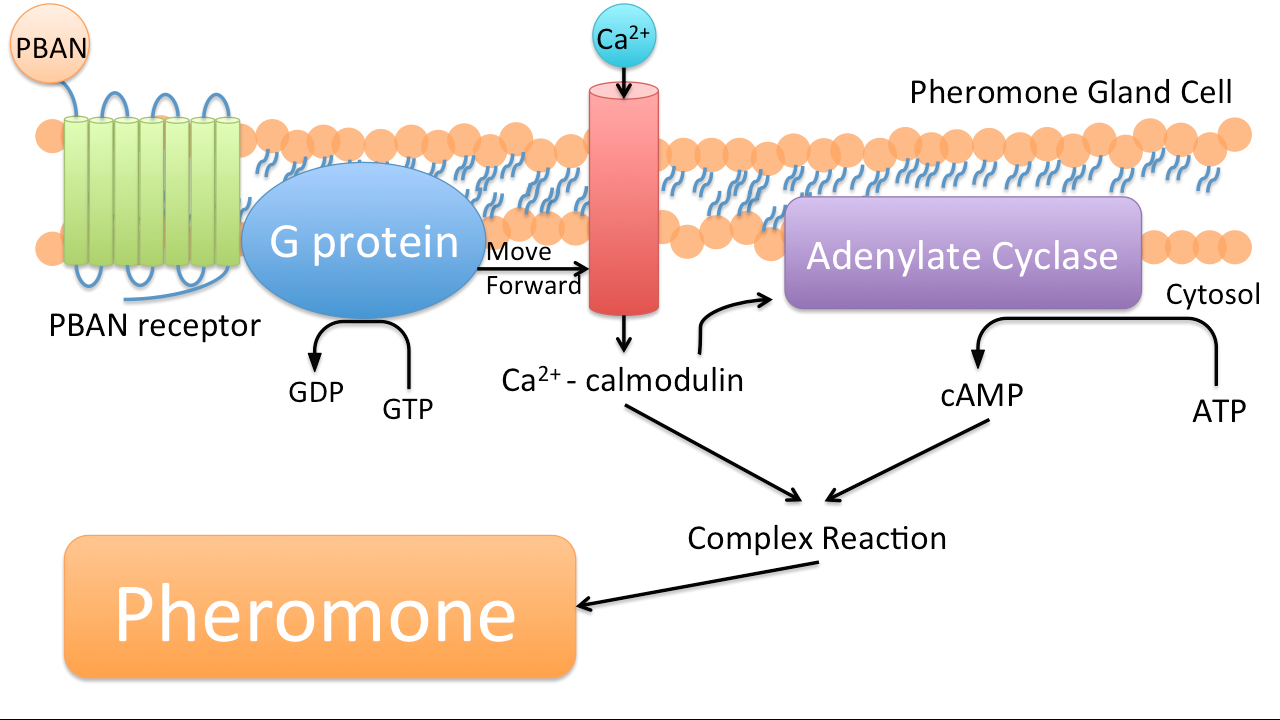
PBAN (Pheromone Biosynthesis Activating Neuropeptide) is one kind of peptides that can activate biosynthesis of pheromones of insects we target. Once a PBAN binds with the G-protein coupled receptor on an insect’s pheromone gland, the signal send by the G-protein coupled receptor activates the kinase and phosphatase, and then kinase and phosphatase can activate enzymes that participate in the biosynthesis of insect pheromone, which will be emitted.
In nature, female insects such as moths release PBAN during mating to stimulate the synthesis of pheromones in order to attract their male counterparts. PBAN can also facilitate the release of non-sex pheromones such as trail pheromones for ants.
Features of PBAN
1. PBAN is species-specific just like pheromones, that means every kinds of insects which can produce pheromone have it's specific PBAN, which can only bind with it's specific receptor and only stimulate the biosynthesis of a specific pheromone.
2. The coding sequence for a PBAN is usually around 100 base pairs. Thus, it is easy for E.coli to express. We can even combine several different PBAN sequences into one BioBrick assembly or even make different regulation for different PBANs.
Effect Testing of PBAN from Reference
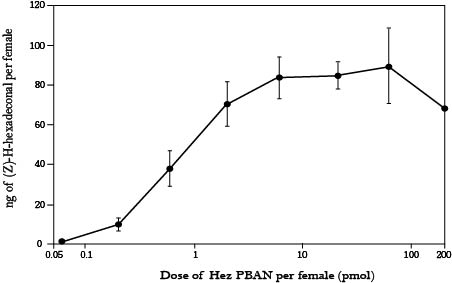
Because we regard PBAN as a leading character in our project, we have to realize what kind of problem we would meet with if we want to make use of PBAN. First, we had to confirm that whether PBAN can work when getting in the moth’s body from outside, we got a paper titled “Pheromonotropic Activity of Naturally Occurring Pyrokinin Insect Neuropeptides (FXPRLamide) in Helicoverpa zea” from ELSEVIER, experiments of the research were conducted with Helicoverpa zea (corn earworm) in this research, researchers injected different amount (from 0.05 pmol to 200 pmol) of PBAN of Helicoverpa zea (named Hez-PBAN) into their bodies directly, and then measured the amount of pheromone produced, the result (fig.1-2-4) showed that PBAN has function as long as it gets in moth’s body, and the 2 pmol dose of Hez-PBAN was the minimum dose required to stimulate production of the maximum amount of pheromone, that revealed we are able to stimulate the production of pheromone with only little amount of PBAN which gets in moth’s body, whether we fed or injected PBAN.
Difficulty of Natural PBAN about Peptidase Degradation
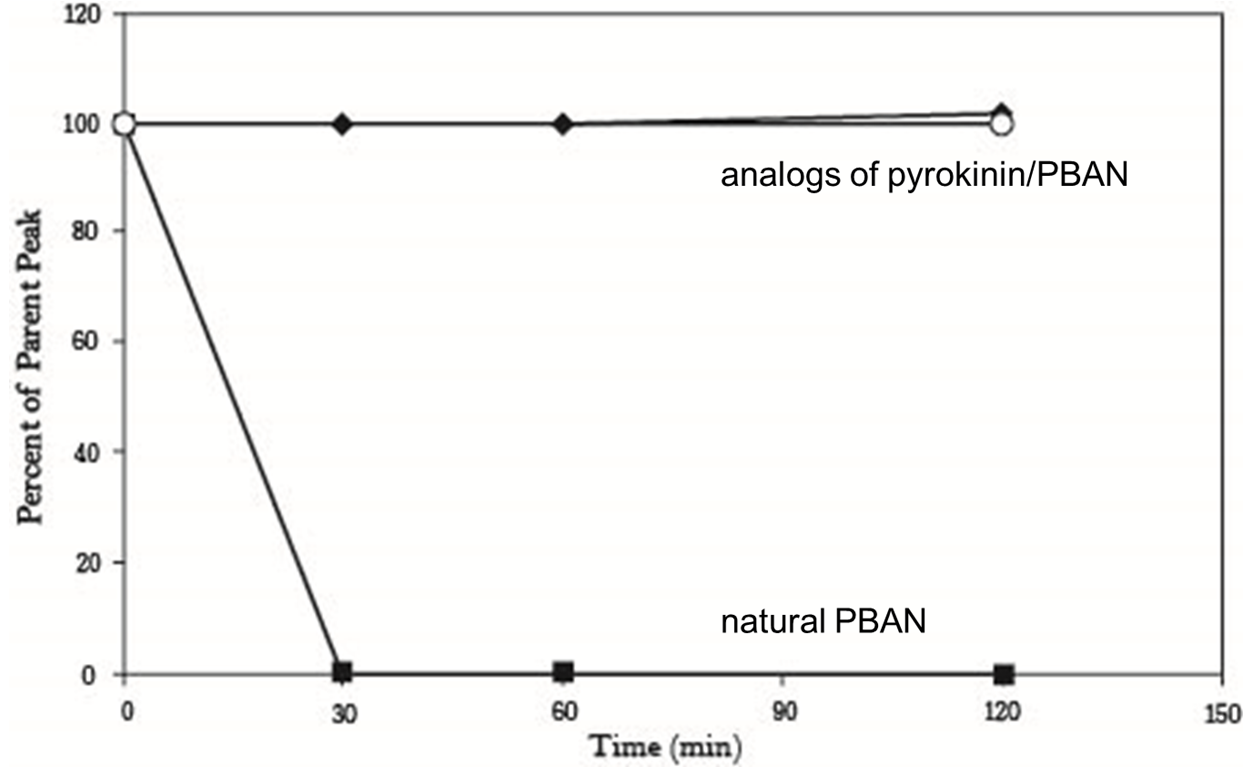
But we still had the second problem, we had known that peptidase in the insect’s body can degrade peptides including PBAN, so the effect of PBAN can’t continue for a long time. In order to solve this problem, we search some former papers and we found another paper from ELSEVIER, it was titled “Enhanced oral availability/pheromonotropic activity of peptidase-resistanttopical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides”, Heliothis virescens was used in this research, researchers produced amphiphilic analogs of pyrokinin/PBAN with chemical synthesis method, they used hydroxyproline (Hyp) and octahydroindole-2-carboxyl (Oic) to replace the Proline (Pro) of the PBAN C-terminal penpapeptides (Phe-Thr-Pro-Arg-Leu-NH2), that made PBAN be able to resist peptidase, they compared the stability of the natural pyrokinin/PBAN analog LPK, and the peptidase-resistant pyrokinin/PBAN analogs (5 nmol was used), we can see that natural PBAN will be reduced over time in the moth’s body (fig.1-2-5).
Our New Idea of using PBAN
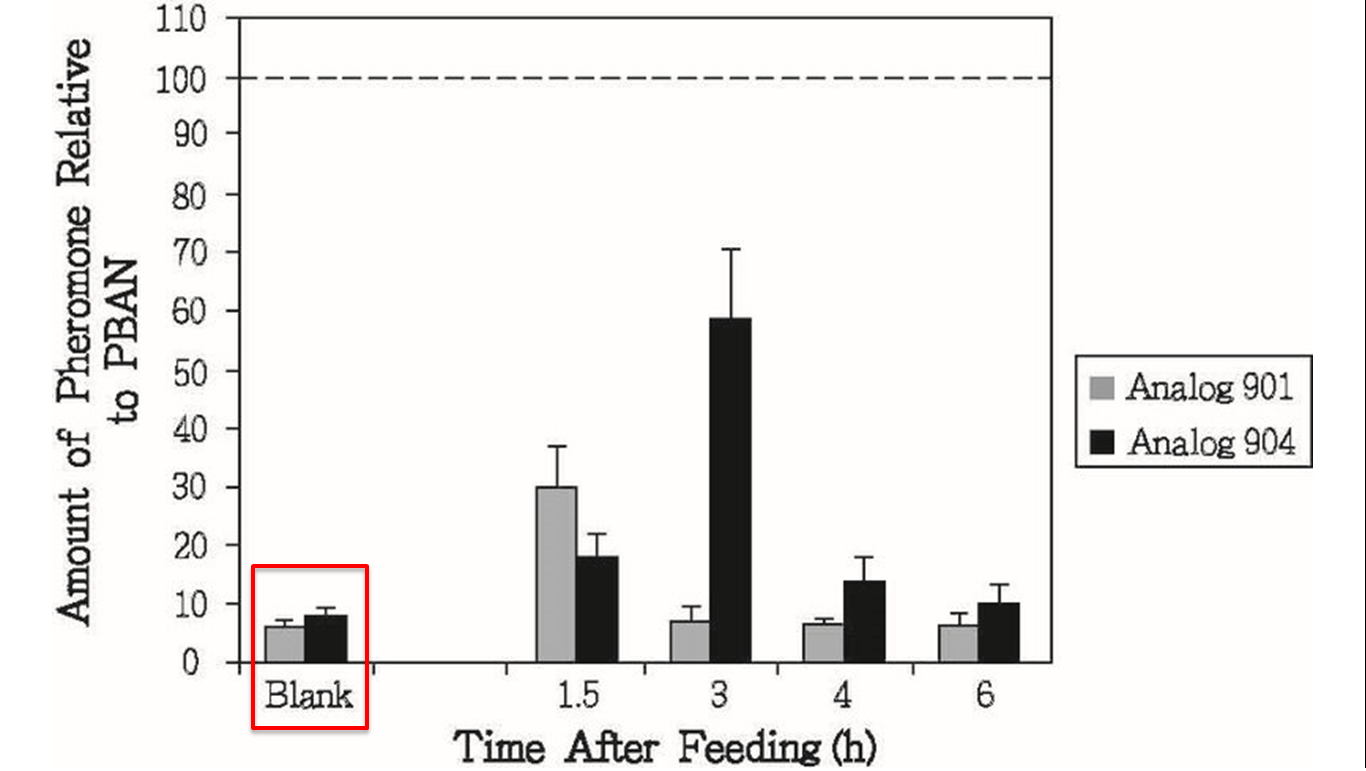
This results above sounded not good for us, but there was other experiment interested us in the same research, researchers also conducted oral test with their PBAN (fig.1-2-6), in this experiment, they fed moths with sugar solution which contained their PBAN analogs, and measure the amount of pheromone produced over time, that gave us inspiration, although natural PBAN can’t be maintained for a long time in moth’s body, we could solve this problem by feeding moths with PBAN continually, after combining this thought with the method of the oral experiment, we figured out an idea, instead of killing all of the moths.
It is very possible that the PBAN in the food will penetrate into moths’ body from the digestive system. Thus, if female moths suck the food, PBAN in the moths’ body will be replenished, which solves the problem that PBAN can not maintain for a long time in the moths’body. As to how we produce PBAN and apply our concept to capture wanted harmful insects, we will explain in the following.
How are we going to use PBAN?
In our project, we will biologically synthesize PBAN with our E.coli. We store the PBAN inside a trapping device (check this out at our Device page). In the device, there will be appropriate lighting and nutrient sources that will attract insects.
Once an insect is attracted into our device and ingests the nutrient sources we provide, it will also inevitably come in contact with our PBAN, which is evenly mixed with the nutrient sources. As the PBAN works its magic and activates the pheromone synthesis of the attracted insect, more of this species of insect’s counterparts will be attracted and later captured.
Owing to the first feature mentioned above, PBAN are species-specific, which means that it doesn't matter if other kind of insect fly into our device and eat PBAN, because the insects we don't want to catch will not be stimulated by PBANs to produce pheromone; our PBAN are only for what we want to catch, and we are sure that our method won't affect other kinds of insects.
PBAN we use
Check photo to see petite sequence.
 |
 |
 |
|---|---|---|
 |
 |
 |
 |
 |
 |
PBAN sequence: LADDMPATPADQEMYRPDPEQIDSRTKYFSPRL
PBAN sequence: LSDDMPARPADQEMYRQDPEQIDSRTKYFSPRL
PBAN sequence: LADDMPATPADQELYRPDPDQIDSRTKUFSPRL
PBAN sequence: GSGEDLSYGDAYEVDEDDHPLFVPRL
PBAN sequence: DASSSNENNSRPPFAPRL
PBAN sequence: LADDTPATPADQEMYRPDPEQIDSRTKYFSPRL
PBAN sequence: LADDMPATMADQEVYRPEPEQIDSRNKUFSPRL
PBAN sequence: QSEAVTSSDEQVYRQDMSPVDGRLKYFSPRL
PBAN sequence: LSEDMPATPADQEMYQPDPEEMESRTRYFSPRL
Reference
- Miriam Altstein, Role of neuropeptides in sex pheromone production in moths, ELSEVIER, Peptides 25 (2004) 1491–1501.
- Ada Rafaeli, Pheromone biosynthesis activating neuropeptide (PBAN): Regulatory role and mode of action, General and Comparative Endocrinology 162 (2009) 69–78
- Ronald J. Nachmana, Peter E.A. Teal, Allison Strey, Enhanced oral availability/pheromonotropic activity of peptidase-resistant topical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides, ELSEVIER, Peptides 23 (2002) 2035–2043 +
- Russell Jurenka1 and Ada Rafaeli, Regulatory role of PBAN in sex pheromone biosynthesis of heliothine moths, frontiers in ENDOCRINOLOGY, published: 10 October 2011 doi: 10.3389/fendo.2011.00046
- Dr. Ashok K. Raina andJulius J. Menn, Pheromone biosynthesis activating neuropeptide: From discovery to current status, Issue Archives of Insect Biochemistry and Physiology, Article first published online: 7 FEB 2005 DOI: 10.1002/arch.940220112
- Man-Yeon Choi and Robert K. Vander Meer, Ant Trail Pheromone Biosynthesis Is Triggered by a Neuropeptide Hormone, PLoS Onev.7(11); 2012PMC3511524
- RELLA L. ABERNATHY, RONALD J. NACHMAN, PETER E. A. TEAL, OKITSUGU YAMASHITAS AND JAMES H. TUMLINSON, Pheromonotropic Activity of Naturally Occurring Pyrokinin Insect Neuropeptides (FXPRLamide) in Helicoverpa zea, ELSEVIER, Peptides Volume 16, Issue 2, 1995, Pages 215–219
Design
Biobrick Design
We searched the DNA sequences of the PBANs from many kinds of insects on NCBI, then contrasted to the amino sequences from papers so that we can select the DNA fragments which directly correspond to gland-stimulating function. By ligating the constitutive promoter (J23101), ribosome binding site (B0034) and PBAN DNA sequence with a terminator (J61048) at the last (we delimit this sequence as basic part), we were able to make E.coli directly produce these PBANs continusely instead of the original complex process of PBAN biosynthesis in insects.
Our biobrick design can be customized to different pest problems. For instance, there is a farm damaged by 3 kinds of moths : Lymantria dispar, Spodoptera litura and Mamestra brassicae. What we only have to do is change the PBAN DNA sequence of 3 basic parts into the PBAN DNA sequence of Lymantria dispar, Spodoptera litura and Mamestra brassicae. Then we assemble these 3 basic parts together. After these pests are attracted into our device, they will ingest these three kinds of PBANs. Subsequently, these 3 species of moths will produce their own pheromones to attract the same species.
It allows us to assemble these basic parts together because the base pairs of these basic parts are small. Therefore, we can assemble different basic parts which contain different PBAN DNA sequences to resolve different pest problems.
Reference
- Torsten Waldminghaus, Nadja Heidrich, Sabine Brantl and Franz Narberhaus .(2007). FourU: a novel type of RNA thermometer in Salmonella . Molecular Microbiology , 65(2): 413–424 DOI:10.1111/j.1365-2958.2007.05794.x
- part BBa_K115002;TUDelft Registry of Standard Biological Parts
Device
Introduction
Since insects behave widely different to the gravity force, we design a device which could catch specific kind of insect species that we want. For example, Agrotis ypsilon Rottemberg and Spodoptera litura falls into the kind of moth that have negative geotaxis. So we made a trap with accessible pathway in the bottom. Once an insect enters the device, it could only goes up and be trapped inside the pyramid. However, after field investigation, we found some insects still escape from the device. Then we came out with a new version trap with doubled layers, inner shell and outer shell.
Mechanism
First, we divide our design chart into two parts-exterior and interior. The exterior is just like the appearance of pyramid, and the interior is used to equip PBAN and bag for pests. When the harmful insects we want to catch eat our PBAN, they will release pheromone, and attract the same species. We can use blue light or the smell of pheromone to attract insect at first. After they go into our device, the design of our device will take advantages of their characteristic that insects always fly high to escape and make them stuck in our device. When we take away the outer shield, the hock on the outer shield will close the bag, and the insects will be caught. In addition, the four tenons at the corner can firm up our device.
Device Design
For the materials of our device, Acrylic is a polymer created when giant carbon molecules combine chemically. Finished acrylic sheet exhibits glass-like qualities – clarity, brilliance, transparency, translucence – but at half the weight and up to 10 times the impact resistance. It can be tinted or colored, mirrored or made opaque. A number of coatings can be applied to a sheet or finished part for performance enhancing characteristics such as scratch resistance, anti-fogging, glare reduction and solar reflective. Because it's a thermoplastic and softens under extremely high temperatures, acrylic can be formed to virtually any shape. Incredibly durable, acrylic is a suitable solution over a broad temperature range, and has superior weathering properties compared to other plastics.
For the process, we searched for some information of our factories, and handed out the design chart. We also went everywhere to buy what we need for our device.
First we use PowerPoint to draw the draft. Because PowerPoint can offer us a drawing tools, we chose it to draw our draft. It did not need a lot of technique. We only have to pay attention to present the brain idea perfectly. In the figure below you can see some of the draft.
Also we use PowerPoint to double check the design. Because if the software has the problems, the manufacturers can open this file to see the right industry with map. It is very important to prevent this thing from happening. In the figure below you can see some of the draft.
Second we use one kind of graphics software ‘‘CorelDRAW’’ to draw the formal design picture. In this software, we can learn how to draw industry with map to cooperate with manufacturers. They usually use it to make design. So we have to learn how to use it software. In the figure below you can see some of the draft.
Now we get the acrylic models finished. We could begin to do our best to assemble and install the inner shell, bottom and outer shell. Although the latch was very expensive, we did it ourselves. The blue light bulb were also assembled by ourselves. Accomplishing our device is very interesting and exciting. Be fun in welding.
Process to assemble our device & main idea of our device design
Outer Shell:
We shaped the device into a pyramid. Its special layout also enriches the device with mysterious colors.
1. Outer shell is composed of 4 triangular acrylic planes which has a trapezoid entrance to let bugs in and release the smell of pheromone produced by the insects which eat our PBAN.
2. There is a hook installed on the outer plane which attaches to the collecting bag inside. When we pull up the outer shell, the bag inside would also be sealed simultaneously so the bugs won’t escape.
Inner Shell:
Similar to the outer one, inner shell are also composed of 4 trapezoid planes and removable from the base. The only different part is that its top is not sealed in order to contain the collecting bag.
1. The inner space can contain a purse-string bag to collect insects we want.
2. 4 rods are assembled like a pound sign on the top to sustain the bag.
3. There would be a PBAN solution placed on the bottom.
4. A blue LED light bulb will be installed around the top of the inner shell plane to attract the first female insect.
Tenon:
A part to stabilize the pyramid.
1. Stabilize 4 corners of the bottom.
2. To make sure the outer shell can combine with the inner one tightly.
Assembling Process
We can assemble the outer shell、inner shell and tenon together to get our completed pyramid device.
Advantages
1.We successfully apply the geotaxis of targeted female moth to trap them in our device, forcing them releasing sex pheromone by our PBAN to attract more same-kind insects.
2.The hook attached to the purse-string bag can seal it simultaneously when we want to remove the outer shell. By doing so, no insects would flee from the bag and safety problems can also be solved.
3.Inner shell is removable so it’s easier to add new PBAN solution.
4.Compared to conventional light bulb, LED bulb is much brighter and conserves more energy. It could powered by battery so it’s also easier to establish.
5.Purse-string bag is cheap and easy to switch.
6.The PBAN system can run day and night. Its function won’t be affected by sunlight.
7.Pyramid is good at looking and can enriches the entire device with a technological feeling.
 "
"