Team:LMU-Munich/Project/Bakillus
From 2014.igem.org
BaKillus - Engineering a pathogen-hunting microbe
Increasing bacterial resistance to classical antibiotics remains a serious threat and urges the development of novel pathogen-killing strategies. Exploiting bacterial communication mechanisms such as quorum sensing is a promising strategy to specifically target certain pathogens. The major aim of this project is the introduction of a genetic circuit enabling Bacillus subtilis to actively detect, attach to, and eventually kill Staphylococcus aureus and Streptococcus pneumoniae. Initially, we will introduce the autoinducer-sensing two-component systems of S. aureus and S. pneumoniae into B. subtilis. to create a pathogen-detecting strain. By utilizing quorum sensing-dependent promoters, we will then trigger pathogen-killing strategies like the production of antimicrobial peptides or biofilm degradation. As a safety measure, a delayed suicide-switch guarantees non-persistence of genetically modified B. subtilis in the absence of pathogens. We envision the use of BaKillus as a smart, cheap and simple-to-use medical device for diagnostics and targeted treatment of multiresistant superbugs.

Sensing
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet. Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet.

Adhesion
Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet. Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet.

Killing
A key aspect of our project is the killing module, which enables BaKillus to dispatch pathogens. Therefore, we implement a variety of killing strategies into our chassis.
One of our killing factors is subtilin, a lanthionine-containing antimicrobial peptide. Just like other lantibiotics, it is active against a wide-range of Gram-positive bacteria by inhibiting the cell wall synthesis and formation of membrane pores. The latter leads to the depolarisation of the membrane potential and thus, to rapid cell death. We decided to use subtilin, as it is natively produced by Bacillus subtilis ATCC6633, a close relative to our chassis and as lantibiotics are considered as alternative to classical antibiotics to which many bacteria already developed resistance.
Another killing factor is lysostaphin, a metalloendopeptidase, which lysates the cellwall of some Staphylococcus species. Since this Enzyme is highly specific, and S. aureus is especially sensitive towards lysostaphin, this is an ideal killing strategy for our Bakillus. In addition, lysostaphin shows no signs of toxicity and a very low potential for allergic reactions in first tests on animals and humans.
Since pathogens are often protected by biofilms and thus not very sensitive towards killing agents, dispersin was integrated as an auxiliary device. This glycosaminhydrolase destroys biofilms of many gram-positive and gram-negative bacteria, including S. aureus, making them more susceptible for antibiotics and other pathogen defeating agents.
Both, lysostaphin and dispersin where developed as Biobricks and tested in B. subtilis by the iGEM Team iGEM12_Lyon_INSA in 2012, and we aim to evaluate the effectiveness of this two S. aureus defeating agents.
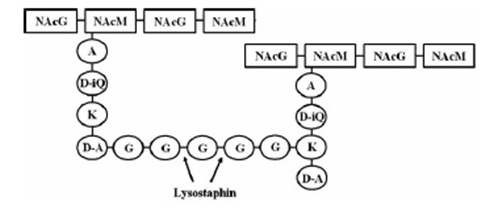
Lysostaphin is a murein-hydrolase, which is naturally produced by Staphylococcus simulans biovar staphylolyticus in order to defeat other, competing Staphylococcus species. It cleaves specifically the pentaglycinbridge between the tetrapeptides of the peptidoglycan in the cell wall and in addition to kill the cells itself, it also dissolves the biofilms of lysostaphin-sensitive bacteria. [A1]
This targeted pentaglycinbridge is a feature of some Staphylococcus species, leading to the high specificity of lysostaphin against S. carnosus, S. epidermidis and Staphylococcus aureus, which is particularly sensitive towards lysostaphin. [A2]
The immunity of S. simulans is accomplished by substitution of the glycine-residues in the cell wall by serine and mediated by the immunity factor Lif [A1]
The preprolysostaphin consists of a signal peptide to mediate the export, 15 tandem repeats of 13 aminoacids length and two protein domains: the peptidase-domain and the C-terminal targeting domain. The signal peptide is cleaved during the export, the tandem repeats are cleaved by an additionally secreted cysteine protease. This repeats are not necessary for correct protein folding or maturation of the protein, they just inhibit protein activity. Once cleaved, the maturated protein is 4.5 fold more active. [A1]
Because of its promising attributes, the usage of lysostaphin as coating for implants [A3] and treatment of S. aureus in the nasal area via cream [A4] is currently in the focus of medical research.
[1] M.C.F. Bastos, H. Ceotto1, M.L.V. Coelho and J.S. Nascimento: Staphylococcal Antimicrobial Peptides: Relevant Properties and Potential Biotechnological Applications in Current Pharmaceutical Biotechnology (2009) S. 38-61 (ABSATZ)
DispersinB (DspB) is a biofilm-dissolving enzyme, specifically a 1,6-beta-N-acetyl-glucosaminidase, produced by Aggregatibacter actinomycetemcomitans, a pathogen, which causes aggressive forms of Parodontitis. [5]
In its natural habitat, A. actinomycetecombitans uses this enzyme to release single cells from the biofilm in order to colonize new habitats. [6]
DspB is active against many bacteria, both gram-positive and gram-negative, and shows some very promising features for its use in medical devices.
It can prevent the growth of biofilms in coated catheters if used in combination with triclosan, and can be used to clear medical devices of biofilms. [9]
[5] Erika Fazekas, Lili Kandra, Gyongyi Gyemant: Model for b-1,6-N-acetylglucosamine oligomer hydrolysis catalysed by DispersinB, a biofilm degrading enzyme, in Carbohydrate Research 363 (2012), S. 7-13
Lysostaphin
[[File:LMU14_Lyst_Background_2.jpg]
Sources:
[2] Jaspal K. Kumar: Lysostaphin: an antistaphylococcal agent in Appl Microbiol Biotechnol (2008) S.555-561 (ABSATZ)
[3] Rohan Satishkumar, Sriram Sankar, Yuliya Yurko, Amy Lincourt, John Shipp, B. Todd Heniford and Alexey Vertegel: Evaluation of the Antimicrobial Activity of Lysostaphin-Coated Hernia Repair Meshes in Antimicrobial Agents and Chemotherapy (2011) (ABSATZ)
[4] John F. Kokai-Kun, Scott M. Walsh, Tanya Chanturiya, and James J. Mond: Lysostaphin Cream Eradicates Staphylococcus aureus Nasal Colonization in a Cotton Rat Model in Antimicrobial Agents and Chemotherapy, 47 (2003)(ABSATZ)
Dispersin
DspB is specific towards its substrate, PNAG, (PGA in E. coli), which is an important component of the extracellular matrix and which can make up to 90% of the dry weight of a biofilm. [7]
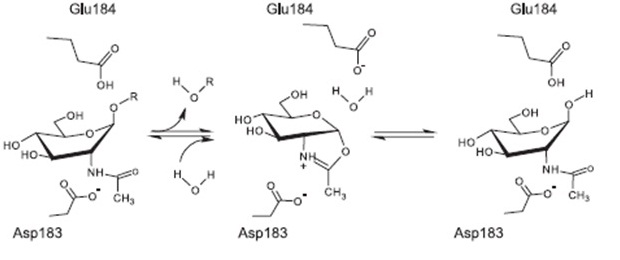
The process of dissolving biofilms is accomplished by cleaving monosaccharide-residues from the polysaccharide, beginning at the non-reducing end of the PNAG. [6]
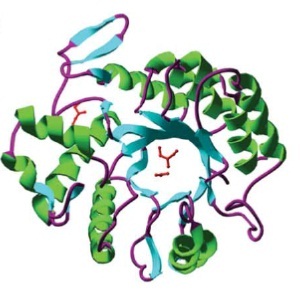
This glycosamin-hydrolase has a typical TIM-barrel-structure, consisting of 8α- and 8β-barrels, with a large cavity in the middle, which is considered to be the substrate-binding pocket. [5]
The currently proposed mechanism of reaction suggests a substrate assisted nucleophilic attack (see chart below. [8]
Sources:
[6] N. RamasubbuL. M. Thomas, C. Ragunath and J. B. Kaplan: Structural Analysis of Dispersin B, a Biofilm-releasing Glycoside Hydrolase from the Periodontopathogen Actinobacillus actinomycetemcomitans, in Journal of Molecular Biology 340 (2005) S. 475-486
[7] Jeffrey B. Kaplan, Kabilan Velliyagounder, Chandran Ragunath, Holger Rohde,
Dietrich Mack, Johannes K.-M. Knobloch, and Narayanan Ramasubbu: Genes Involved in the Synthesis and Degradation of Matrix Polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae Biofilms, in JOURNAL OF BACTERIOLOGY, 186 (2004) S.8213-8220
[8] Suba G. A. Manuel, Chandran Ragunath, Hameetha B. R. Sait, Era A. Izano, Jeffrey B. Kaplan and Narayanan Ramasubbu: Role of active-site residues of dispersin B, a biofilm-releasing b-hexosaminidase from a periodontal pathogen, in substrate hydrolysis, in The FEBS journal (2007)
[9] Darouiche RO, Mansouri MD, Gawande PV, et al. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J Antimicrob Chemother 2009; 64: 88-93
Suicide
Bacillus subtilis has the ability to differentiate into several sub-populations during stationary phase, influenced by the regulation of different gene-clusters. As one of the options, a sub-population can become a so called "Cannibalism" toxin producer - a process which is driven by the master regulator Spo0A. Those cannibalistic cells kill their non-cannibalistic mates and feed on the nutrients set free upon lysis.


Hi there!
Welcome to our Wiki! I'm BaKillus, the pathogen-hunting microbe, and I'll guide you on this tour through our project. If you want to learn more about a specific step, you can simply close the tour and come back to it anytime you like. So let's start!

What's the problem?
First of all, what am I doing here? The problem is, pathogenic bacteria all around the world are becoming more and more resistant against antimicrobial drugs. One major reason for the trend is the inappropriate use of drugs. With my BaKillus super powers, I want to reduce this misuse and thus do my part to save global health.

Sensing of pathogens
To combat the pathogenic bacteria, I simply eavesdrop on their communication. Bacteria talk with each other via quorum sensing systems, which I use to detect them and trigger my responses.

Adhesion
The more specific and effective I can use my powers, the lower the danger is of provoking new resistance development. So I catch pathogens whenever I get hold of them and stick to them until my work is done.

Killing
Talking about my work - killing pathogens is finally what I am made for. In response to quorum sensing molecules of the pathogens, I export a range of antimicrobial substances leading to dissipation of biofilms and the killing of the targeted bacteria.

Suicide switch
When the job is done and all the bad guys are finished, you don't need a super hero anymore. So after fulfilling my work I say goodbye to the world by activating my suicide switch.

Application
Of course I'm not only a fictional hero, but a very real one. In two different prototypes, I could be used for diagnosis or treatment of pathogen-caused diseases. However, there is still a whole lot of regulational and economical questions that have to be answered before.

See you!
So now you know my short story - and it is time for me to return to my fight for a safer world. Feel free to take a closer look on my super powers, the process of my development or the plans for a medical application.
 "
"