Team:Paris Saclay/Notebook/July/29
From 2014.igem.org
(→C - Salicylate Inducible Suppressing System) |
(→Electrophoresis) |
||
| Line 213: | Line 213: | ||
====Electrophoresis==== | ====Electrophoresis==== | ||
| + | [[File:LU000107a.jpg|right|300px]] | ||
| + | [[File:RL007744.jpg|right|300px]] | ||
''by Fabio'' | ''by Fabio'' | ||
| - | ''''Process A'''' | + | First we tested both products of the digestions in order to verify the success of the reaction (''Process A''), which remained for 25 minutes in 100V. Once the sizes of the digestion's products confirmed, we did the ''Process B'' in order to segregate a good amount of DNA to the next step. |
| + | |||
| + | '''''Process A''''' | ||
# BBa_J61051 | # BBa_J61051 | ||
| - | # | + | # BBa_K228001 |
| + | |||
| + | '''''Process B''''' | ||
| + | # BBa_J61051 ''strains from the bottom band of '''process A''''' | ||
'''Results:''' | '''Results:''' | ||
| + | *'''A''' #1: Success, we can clearly see the vector and the core of the BioBrick after the digestion. | ||
| + | *'''A''' #2: Success, the digested BioBrick was cut one time and has the expected size. | ||
| + | *'''B''': Success, we have a very good concentration of the BBa_J61051's core. | ||
| - | + | [https://2014.igem.org/Team:Paris_Saclay/Protocols/BioBrick_Assembly#Segregate_process BioBrick Assembly - Segregate Process Protocol] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===D - Lemon scent=== | ===D - Lemon scent=== | ||
Revision as of 12:58, 1 August 2014
Contents |
Tuesday 29th July
Lab Work
A - The frame coli Odor free
PCR
by Romain
Strains used: E. coli MG1655Z1 and E. coli MG1655, and oligonucleotides used: FTR-Apra-F and FTR-Apra-R.
Protocol
Add into a PCR tube the following:
| Component | For a total volume of 50μl |
|---|---|
| H2O | 27.25μl |
| Green GoTaq buffer 5X | 10μl |
| dNTPs | 1μl |
| FTR-Apra-F | 2μl |
| FTR-Apra-R | 2μl |
| DMSO | 1.5μl |
| MgCl2 | 4μl |
| Bacterial culture | 2μl |
| Green GoTaq enzyme | 0.25μl |
Tube was placed in PCR machine with the following parameters.
| Cycle step | Temperature | Time | Cycle |
|---|---|---|---|
|
Initial denaturation |
95°C |
5 min |
1 |
| Denaturation | 95°C | 30 s | 30 |
| Annealing | 60°C | 30 s | 30 |
| Extension | 72°C | 2 min | 30 |
| Final extension | 72°C | 5 min | 1 |
| Final extension | 12°C | hold | 1 |
The different bacterial cultures in each tubes:
- Tube 1: MG1655Z1 10I
- Tube 2: MG1655 100II
- Tube 3: MG1655 50I
- Tube 4: MG1655 100I
- Tube 5: MG1655Z1 10II
- Tube 6: MG1655 50II
- Tube 7: MG1655Z1 10I positive control with plasmid
Results of the PCR: The electrophoresis revealed nothing
New PCR with different enyme.
Strains used: MG1655 and MG1655Z1, and oligonucleotides used: FTR-Apra-F and FTR-Apra-R.
Protocol
Add into a PCR tube the following:
| Component | For a total volume of 50μl |
|---|---|
| H2O | 37.25μl |
| DreamTaq buffer 10X | 5μl |
| dNTPs | 1μl |
| FTR-Apra-F | 1μl |
| FTR-Apra-R | 1μl |
| DMSO | 2.5μl |
| Bacterial culture | 2μl |
| DreamTaq enzyme | 0.25μl |
Tube was placed in PCR machine with the following parameters.
| Cycle step | Temperature | Time | Cycle |
|---|---|---|---|
|
Initial denaturation |
95°C |
5 min |
1 |
| Denaturation | 95°C | 30 s | 30 |
| Annealing | 60°C | 30 s | 30 |
| Extension | 72°C | 2 min | 30 |
| Final extension | 72°C | 5 min | 1 |
| Final extension | 12°C | hold | 1 |
- Tube 1: MG1655Z1 10I
- Tube 2: MG1655 100II
- Tube 3: MG1655 50I
- Tube 4: MG1655 100I
- Tube 5: MG1655Z1 10II
- Tube 6: MG1655 50II
- Tube 7: MG1655Z1 10I positive control with plasmid
Results of the PCR: The electrophoresis revealed successfully the DNA!
C - Salicylate Inducible Suppressing System
Digestion
by Fabio
After the experiments done the 28th July, we are sure to manipulate the right plasmid from both BioBricks and also that we have a good concentration of them. Now it's time to start the [http://parts.igem.org/Help:Assembly/3A_Assembly Assembly procedure] in order to concatenate both BioBricks.
- BioBrick BBa_J61051 (Salicylate promoter + NahR) as Part A
- BioBrick BBa_K228001 (RNA suppressor) as Part B
TODO: Illustration of the process
BioBrick Assembly - Digest Reaction Protocol
Electrophoresis
by Fabio
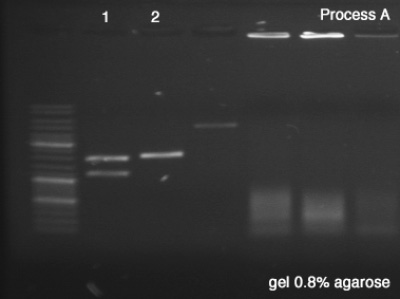
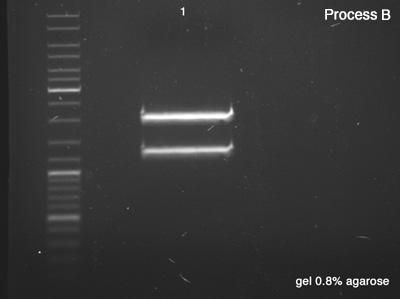
First we tested both products of the digestions in order to verify the success of the reaction (Process A), which remained for 25 minutes in 100V. Once the sizes of the digestion's products confirmed, we did the Process B in order to segregate a good amount of DNA to the next step.
Process A
- BBa_J61051
- BBa_K228001
Process B
- BBa_J61051 strains from the bottom band of process A
Results:
- A #1: Success, we can clearly see the vector and the core of the BioBrick after the digestion.
- A #2: Success, the digested BioBrick was cut one time and has the expected size.
- B: Success, we have a very good concentration of the BBa_J61051's core.
BioBrick Assembly - Segregate Process Protocol
D - Lemon scent
Transformation of electrocompetent cells
by Arnaud & Terry
Strain used: DY330. Plasmid used: pJBEI-6409 (code for enzymes to produce limonene from glucose in E. coli)
Make 2 électroporations in cold electroporation cuvettes:
- A control cuvette(without DNA): 50µl of DY330.
- A second cuvette: 50µl of DY330 culture + 1µl of pJBEI-6409 plasmid.
Electroporation : 2500V, 132W, 40µF.
After that, add 1ml of cold LB in each cuvette and transfer in 2 tubes to incubate during 1 to 2h at 30°C.
Spread on 4 dishes LB + Cm:
- 100µl of control (without plasmid)
- 50µl of transformed DY330 with pJBEI-6409
- 100µl of transformed DY330 with pJBEI-6409
- The rest of transformed DY330 with pJBEI-6409 (concentrate in approximately 150µl)
Incubate for the weekend at 30°C.
PCR of BBa_K762100
by Sean
Oligonucleotides used: iPS66, iPS67
Oligonucleotides were diluted twice from 100µM to 50µM.
Protocol
Add into a PCR tube the following:
| Component | For a total volume of 50μl |
|---|---|
| H2O | 35.5μl |
| Phusion buffer 5X | 10μl |
| dNTPs | 1μl |
| iPS66 | 1μl |
| iPS67 | 1μl |
| BBa_K762100 | 1μl |
| Phusion DNA polymerase | 0.25μl |
Tube was placed in PCR machine with the following parameters.
| Cycle step | Temperature | Time | Cycle |
|---|---|---|---|
|
Initial denaturation |
98°C |
1 min |
1 |
| Denaturation | 98°C | 15 s | 25 - 30 |
| Annealing | 55°C | variable | 25 - 30 |
| Extension | 72°C | 45 s | 25-30 |
| Final extension | 72°C | 10 min | 1 |
| Final extension | 8°C | hold | 1 |
Members there:
- Instructors and advisors: Alice, Solenne and Sylvie.
- Students: Arnaud, Fabio, Romain, Sean and Terry.
 "
"