Team:SUSTC-Shenzhen/Notebook/HeLaCell/A-B-Toxin-transfect
From 2014.igem.org
| Line 45: | Line 45: | ||
{{SUSTC-Image|wiki/images/e/e6/SUSTC-Shenzhen-A-B-Toxin-figure1.jpg|Figure 1.}} | {{SUSTC-Image|wiki/images/e/e6/SUSTC-Shenzhen-A-B-Toxin-figure1.jpg|Figure 1.}} | ||
The cells are observed 12 hours after transfection (Figure 1). Although when excited with blue light, the cells shows some red pots in both the control and the three test groups. But it is probably the noise result from the dead cells and the culture medium. So in the next day, the medium is changed with PBS and another set of photo is taken (Figure 2). | The cells are observed 12 hours after transfection (Figure 1). Although when excited with blue light, the cells shows some red pots in both the control and the three test groups. But it is probably the noise result from the dead cells and the culture medium. So in the next day, the medium is changed with PBS and another set of photo is taken (Figure 2). | ||
| - | {{SUSTC-Image|wiki/images/ | + | {{SUSTC-Image|wiki/images/a/a8/SUSTC-Shenzhen-A-B-Toxin-figurex.jpg|Figure 2.}} |
3 days after transfection, the cells are observed again (figure 3). | 3 days after transfection, the cells are observed again (figure 3). | ||
{{SUSTC-Image|wiki/images/a/a8/SUSTC-Shenzhen-A-B-Toxin-figure3.jpg|Figure 3.}} | {{SUSTC-Image|wiki/images/a/a8/SUSTC-Shenzhen-A-B-Toxin-figure3.jpg|Figure 3.}} | ||
Unfortunately, we have not observed the red fluorescence of mCherry under fluorescence microscope until now. However, the cells transfected with TEG protein grows well. Before transfection, it is still been worried that the protein solution make affect the cell growth. | Unfortunately, we have not observed the red fluorescence of mCherry under fluorescence microscope until now. However, the cells transfected with TEG protein grows well. Before transfection, it is still been worried that the protein solution make affect the cell growth. | ||
| - | + | ==The second transfection== | |
The proteins are purified again, and the second transfection is done. | The proteins are purified again, and the second transfection is done. | ||
| - | ==New materials== | + | ====New materials==== |
*TEG protein solution (4μg/mL) | *TEG protein solution (4μg/mL) | ||
*GD5 protein solution (15μg/mL) | *GD5 protein solution (15μg/mL) | ||
| + | |||
| + | ===Procedure=== | ||
| + | The procedure is the same as the first time, unless the amount of DNA is doubled and the volume of protein solution is doubled in the transfection system (There is some impurities in the protein solution, so the concentration of the TEG or GD5 proteins measured is supposed to be higher than they what they should be). What’s more, a positive control group cells transfected with lipofetamine 3000 reagent is added. | ||
| + | {{SUSTC-Image||}} | ||
| + | Then the cells was observed under fluorescence microscope in the following days (Figure 4). The mCherry signal is observed in the cells transfected by lipofectamine 3000 but not in the cells transfected by TEG or GD5. | ||
| + | The cells are read by flow cytometry 2 days after transfection (Figure 5). It seems that the second transfection do not succeed. | ||
| + | {{SUSTC-Image||}} | ||
| + | ==The third transfetion(16/10)== | ||
| + | New TEG protein are obtained again, the second transfection is repeated with the new TEG protein. What's more, Considering the EGF in the FBS antagonizes the TEG protein, this time the cells are cultured in DMEM without FBS. Unfortunately, this time the cells added protein solution died about 15 hours after transfection. | ||
| + | ==The forth transfection(17/10)== | ||
| + | The forth TEG protein are obtained. The proteins used in the second and third transfection are used to do transfection again. | ||
| + | |||
Revision as of 03:46, 18 October 2014
Notebook
Elements of the endeavor.
Contents |
Transfection of cells with A-B toxin.
2014/10/3 Introduce gRNA with TEG/GD5 protein!Introduction:
TEG is a fusion protein designed for intracellular delivery of DNA by binding to the EGF receptor on human cells. It incorporates GAL4 which can bind to plasmids with UAS sequence, TGF-α which is a ligand of human EGF receptor and sequences encoding the translocation domain of Pseudomonas exotoxin A. GD-5 is another fusion protein similar to TEG but it mediates the DNA transfection by binding to ErbB2 receptor on human cells. Now the two proteins have been purified and refolded, and they will be used to transfect the cells to examine the transfection efficiency of the proteins.
Materials:
Plasmids:
- Endo-free extracted 0XUAS encoding gRNA for EGFP (1006.1ng/μL)
- Endo-free extracted 2XUAS encoding gRNA for EGFP (856.8ng/μL)
- Endo-free extracted 5XUAS encoding gRNA for EGFP (969.2ng/μL)
- Endo-free extracted 7XUAS encoding gRNA for EGFP (1095.5ng/μL)
Cells and culture conditions:
- Cells: HeLa cells stably transfected with EGFP gene located before Bla resistance gene and sharing the same promoter with the resistance gene, and Cas 9 gene whose expression is under the control of Teton.
- Medium before transfection:
- DMEM + 10% FBS, 2mM L-glutamine, 100 units/mL penicillin, 100μg/mL streptomycin, 2.0 μg/mL puromycin, 2.8 μg/mL blasticidin.
- Medium during transfection: DMEM + 10% FBS, 2mM L-glutamine.
- Medium after transfection: DMEM + 10% FBS, 2mM L-glutamine, 100 units/mL penicillin, 100μg/mL streptomycin, 2.0 μg/mL puromycin.
- TEG protein solution (10 μg/mL, 38KDa)
Procedures:
Transfection of cells with GD5-polyplex
- (Oct. 3rd)Preparation of the buffer:
- 50X KCl(2.5M): 3.73g KCl dissolved in 20mL ddH20;
- 100X MgCl2(5M):1.02g MgCl2 dissolved in 10mL ddH2O;
- 88.3mM ZnCl2;
- 30mL Tris buffer, pH 7.5, 50mM KCl, 5mM MgCl2 and 5mM ZnCl2;
- 0.1817g Tris dissolved with 29mL ddH2O + 600μL 2.5M KCl + 300μL 5M MgCl2 + 34μL 88.3mM ZnCl2.
- 20X (about 500nM)poly-lysine HBr: 25mg poly-lysine dissolved with 5mL PBS, then take 200μL of the solution and add PBS to get a total volume of 10mL.
- To get cell culture medium with final DNA 0.5nM (5000bp plasmid with UAS sequence, about 1.69μg/mL), poly-lysine HBr 25nM, protein about 50nM:
- 100μL protein solution (10 μg/mL, 38KDa) + 0.35μL DNA (2X UAS, 5XUAS, and 7XUAS, all with the concentration about 1μg/μL), incubate for 15min at room temperature.Slowly add 25μL 500nM poly-lysine HBr to the mixture of DNA and Protein, then incubate for another 15 min at RT.For the control group, use Tris buffer, pH 7.5, 50mM KCl, 5mM MgCl2 and 5mM ZnCl2 to replace the TEG protein solution.
- Add protein-DNA to the cells(about 80-90% confluence) and incubation for about 24hours, then change medium.
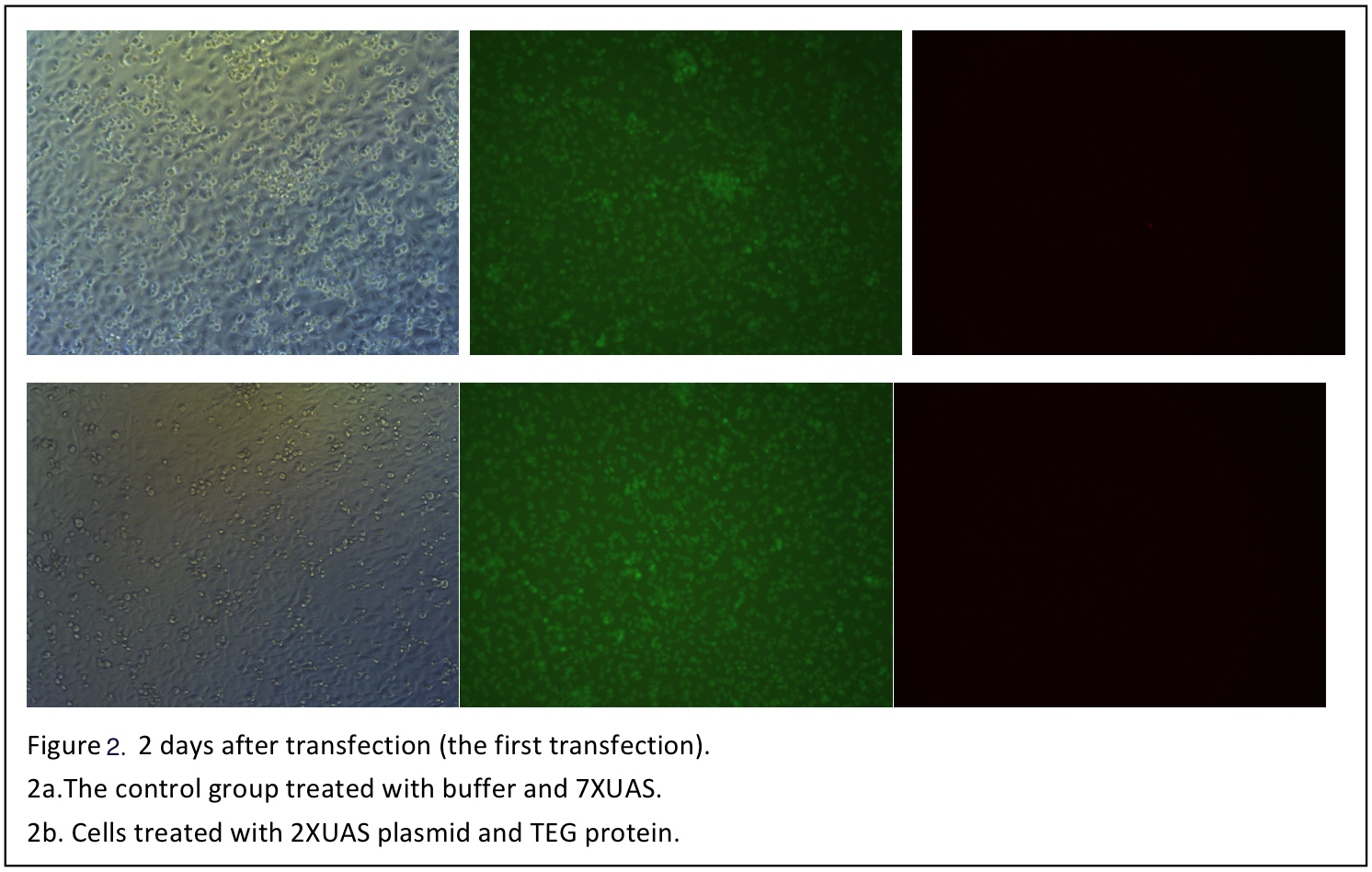
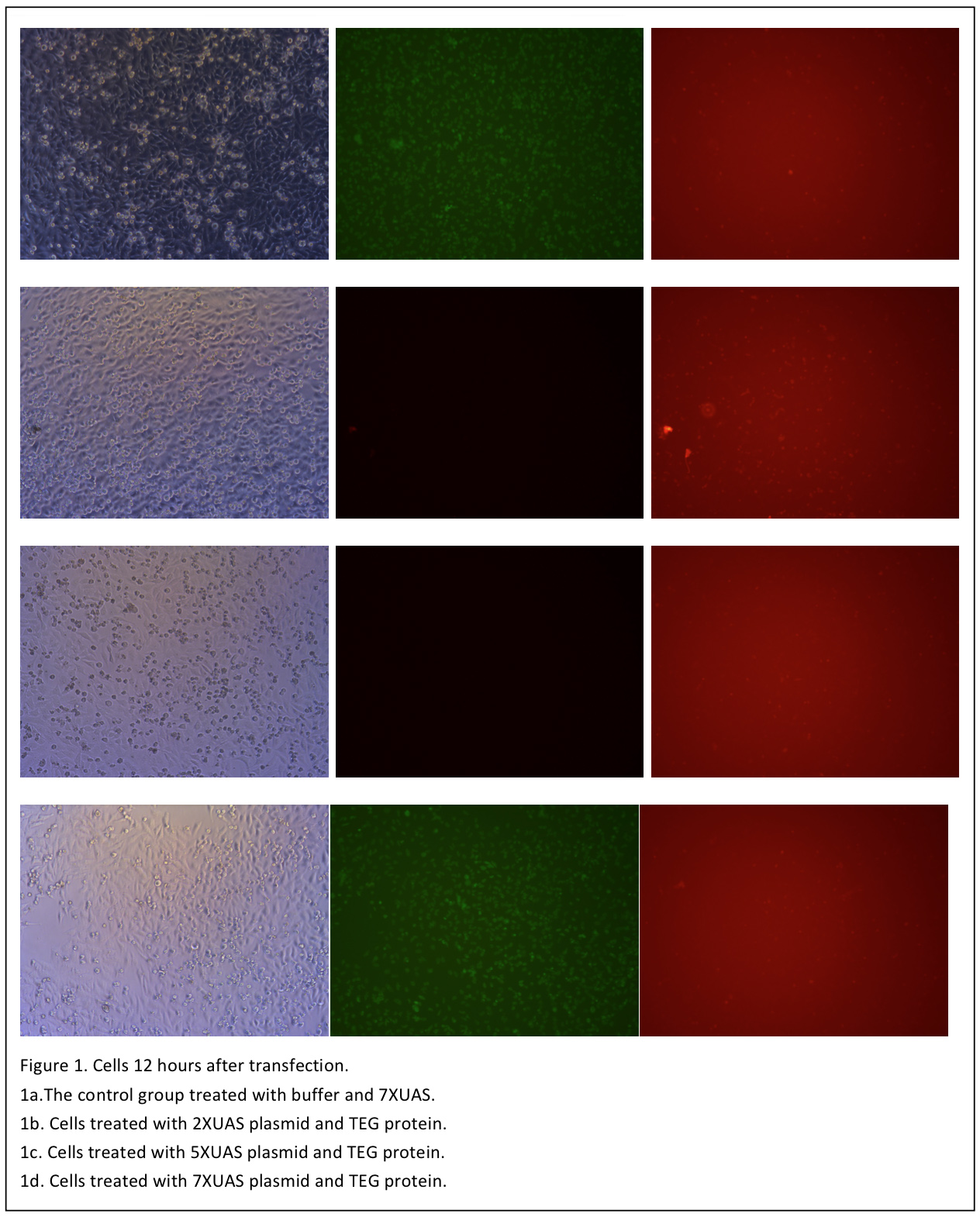 The cells are observed 12 hours after transfection (Figure 1). Although when excited with blue light, the cells shows some red pots in both the control and the three test groups. But it is probably the noise result from the dead cells and the culture medium. So in the next day, the medium is changed with PBS and another set of photo is taken (Figure 2).
The cells are observed 12 hours after transfection (Figure 1). Although when excited with blue light, the cells shows some red pots in both the control and the three test groups. But it is probably the noise result from the dead cells and the culture medium. So in the next day, the medium is changed with PBS and another set of photo is taken (Figure 2).
 3 days after transfection, the cells are observed again (figure 3).
3 days after transfection, the cells are observed again (figure 3).
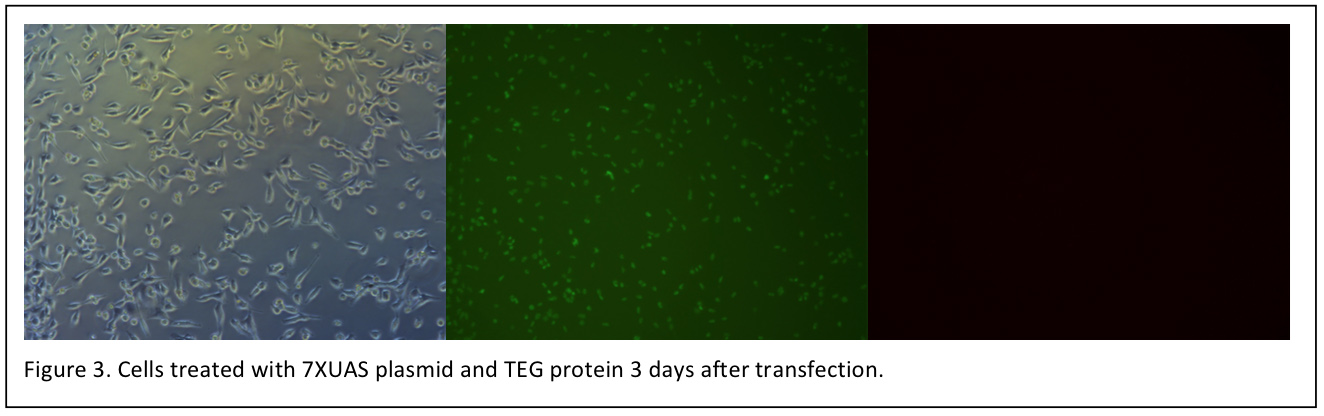 Unfortunately, we have not observed the red fluorescence of mCherry under fluorescence microscope until now. However, the cells transfected with TEG protein grows well. Before transfection, it is still been worried that the protein solution make affect the cell growth.
Unfortunately, we have not observed the red fluorescence of mCherry under fluorescence microscope until now. However, the cells transfected with TEG protein grows well. Before transfection, it is still been worried that the protein solution make affect the cell growth.
The second transfection
The proteins are purified again, and the second transfection is done.
New materials
- TEG protein solution (4μg/mL)
- GD5 protein solution (15μg/mL)
Procedure
The procedure is the same as the first time, unless the amount of DNA is doubled and the volume of protein solution is doubled in the transfection system (There is some impurities in the protein solution, so the concentration of the TEG or GD5 proteins measured is supposed to be higher than they what they should be). What’s more, a positive control group cells transfected with lipofetamine 3000 reagent is added.
Then the cells was observed under fluorescence microscope in the following days (Figure 4). The mCherry signal is observed in the cells transfected by lipofectamine 3000 but not in the cells transfected by TEG or GD5.
The cells are read by flow cytometry 2 days after transfection (Figure 5). It seems that the second transfection do not succeed.
The third transfetion(16/10)
New TEG protein are obtained again, the second transfection is repeated with the new TEG protein. What's more, Considering the EGF in the FBS antagonizes the TEG protein, this time the cells are cultured in DMEM without FBS. Unfortunately, this time the cells added protein solution died about 15 hours after transfection.
The forth transfection(17/10)
The forth TEG protein are obtained. The proteins used in the second and third transfection are used to do transfection again.
 "
"