Team:Brasil-SP/Project/DetectionModule
From 2014.igem.org
| Line 1: | Line 1: | ||
{{:Team:Brasil-SP/Templates/Header|headersrc=TheProjectBRASILSP.png}} | {{:Team:Brasil-SP/Templates/Header|headersrc=TheProjectBRASILSP.png}} | ||
| - | < | + | <h1>Detection Module</h1> |
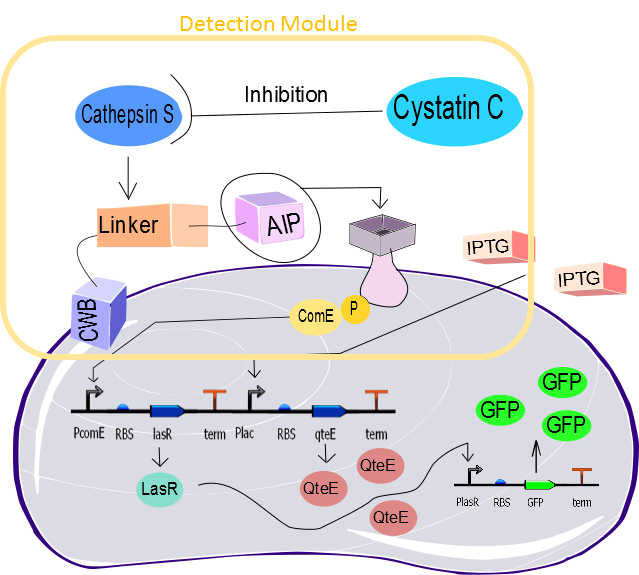
| + | The genetic circuit that is being developed by the Brasil-SP team is a biodetection system designed for Cystatin C (Cys C). We will use the Quorum Sensing bacterial recognition system (QS), based on communication between bacteria. This system consists in the recognition and release of substances that diffuse through the environment; those substances are called autoinducers. The autoinducers are responsible for the activation of their own synthesis, allowing the bacterial cells to respond appropriately to cell population growth. Thus, it enables the control of specific genes expression, which are only activated when a certain cell population density is reached; as a result the bacteria behave coordinately (MILLER and BASSLER, 2001). | ||
| + | In our biosensor, the Quorum Sensing substance is called AIP and its receiver is called ComD. Both AIP and ComD are anchored in the cell membrane. The AIP is attached to a linker that can be cleaved specifically by the protease cathepsin S. After cleavage and release, the AIP binds to its receptor triggering the phosphorylation of ComE, an intracellular signalling molecule, which binds to a specific promoter initiating the expression of the downstream gene (lasR). The cathepsin S can have its proteolytic activity inhibited by Cys C, which would affect all the cascade described previously. Having said that, the Cys C concentrations will be detected through the variation of the cathepsin S protease activity, which ultimately causes regulates LasR production. In the Diagnosis section we describe how the bacteria will be capable of processing this information cariied by the LasR concentration to discriminate between normal and increased Cys C concentration. | ||
| + | <!--<p> One of the crucial steps in this project is to establish a threshold between normal and abnormal Cystatin C level. This threshold will be established using the threshold system constituted by Pseudomonas aeruginosa QteE and LasR genes. In P. aeruginosa, the expression of QteE is controlled by a constitutive promoter while LasR is indirectly induced by AIP through the action of ComE. We will also use a promoter inducible by isopropyl β-D-1-thiogalactopyranoside (IPTG) to control QteE transcription and translation. The QteE protein destabilizes LasR protein so that LasR cannot induce the expression of downstream genes. While the concentration of QteE is equal or greater than LasR, almost all LasR proteins are destabilized. Thus, QteE creates a barrier to cell response. By controlling expression levels of QteE gene, we manipulate the barrier to standardize the AIP concentration, that it is closely related to the Cystatin C concentration. If the concentration of LasR is high enough to overcome the barrier imposed by QteE, the LasR promoter is induced and the reporter gene Green Fluorescent Protein (GFP) is transcribed. However, the LasR and QteE proteins are part of the Quorum Sensing system of gram-negative bacteria, while our genetic circuit will be built in Bacillus subtilis, a gram-positive bacteria. To guarantee the appropriate folding of LasR protein and the induction of the promoter by LasR, substances called HSLs (homoserine lactone) must be added to the system. HSLs are intermediate products of a pathway triggered by the LasI gene. As these intermediates are found in Bacillus subtilis, the activation of transcription by a constitutive promoter meets the demand for HSLs. On the other hand, the aiiA gene in gram-positive bacteria leads to HSLs degradation. In order to guarantee a good performance of our circuit, the aiiA gene will be knocked out.</div></p>--> | ||
| + | |||
| + | |||
| + | {{:Team:Brasil-SP/Templates/Image | image=Circuit_detection.png | alignment=center | caption= | size=500px}} | ||
| + | |||
| + | |||
| + | <h2> Reference</h2> | ||
| + | #MILLER MB, BASSLER BL. Quorum sensing in bacteria. <b>Annual Review of Microbiology </b>, 2001, 55:165-99.<br><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{{:Team:Brasil-SP/Templates/Footer}} | {{:Team:Brasil-SP/Templates/Footer}} | ||
Latest revision as of 00:16, 18 October 2014
Detection Module
The genetic circuit that is being developed by the Brasil-SP team is a biodetection system designed for Cystatin C (Cys C). We will use the Quorum Sensing bacterial recognition system (QS), based on communication between bacteria. This system consists in the recognition and release of substances that diffuse through the environment; those substances are called autoinducers. The autoinducers are responsible for the activation of their own synthesis, allowing the bacterial cells to respond appropriately to cell population growth. Thus, it enables the control of specific genes expression, which are only activated when a certain cell population density is reached; as a result the bacteria behave coordinately (MILLER and BASSLER, 2001).
In our biosensor, the Quorum Sensing substance is called AIP and its receiver is called ComD. Both AIP and ComD are anchored in the cell membrane. The AIP is attached to a linker that can be cleaved specifically by the protease cathepsin S. After cleavage and release, the AIP binds to its receptor triggering the phosphorylation of ComE, an intracellular signalling molecule, which binds to a specific promoter initiating the expression of the downstream gene (lasR). The cathepsin S can have its proteolytic activity inhibited by Cys C, which would affect all the cascade described previously. Having said that, the Cys C concentrations will be detected through the variation of the cathepsin S protease activity, which ultimately causes regulates LasR production. In the Diagnosis section we describe how the bacteria will be capable of processing this information cariied by the LasR concentration to discriminate between normal and increased Cys C concentration.
Reference
- MILLER MB, BASSLER BL. Quorum sensing in bacteria. Annual Review of Microbiology , 2001, 55:165-99.
 "
"