Team:Nagahama project
From 2014.igem.org
(→Medium) |
(→Medium) |
||
| Line 96: | Line 96: | ||
===Trypton Broth=== | ===Trypton Broth=== | ||
| - | ===Wash | + | ===Wash medium=== |
| - | ===Chemotaxis | + | ===Chemotaxis medium=== |
| + | |||
| + | ===Synthesis medium=== | ||
| + | |||
| + | ===LB medium=== | ||
| + | |||
| + | ===2×YT medium=== | ||
| + | |||
| + | ===Swarming Agar=== | ||
==Aspartate synthesis== | ==Aspartate synthesis== | ||
Revision as of 02:55, 16 October 2014
| ||||||||||||||
Contents |
Our Project
Modeling
We consigned modeling of our project to
UT-Tokyo.They readily compiled with our requests. Their model is ideal.We really appreciate their jobs We wish a friendly relationship with UT-Tokyo.Thank you so much!! The detail of their jobs are lists below!!
Method
Medium
Trypton Broth
Wash medium
Chemotaxis medium
Synthesis medium
LB medium
2×YT medium
Swarming Agar
Aspartate synthesis
E.coli K12 transformed with CdP-R.B.S-AspA-d.Ter (BBa_K1342001) previous cultured with cadmium in LB medium (250μM) in 37℃ for 12hr at 120rpm. Adjust Cell mass (OD1.0) and therefor centrifuged 4000rpm for 20 min. Cell pellets ware activated in synthesis medium in 37℃ for 2hr at 120rpm/min.
SDS PAGE
・Preculture E.coli holding a plasmid containing a target gene or nomal E.coli.
・Measure OD600 0.6-1.0
・the expression of fusion protein by isopropylthio-β-D-galactoside (IPTG) and Cd2+ soln.
・Transfer a sample a 200 µl in a microcentrifuge tube
・Centrifuge at 13,000rpm for a minute at 4℃
・Discard supernatant quantitative
・Store pellet at -20 °C
・Thaw pellet and resupend in Sample Buffer (100 µL 1xSample Buffer per samples)
・Heat for 5 minutes at 98 °C
・Centrifuge at 13,000rpm for 10 minutes at 4℃
・Transfer supernatant to a new microcentrifuge tube
・Analyze samples by SDS-PAGE.(Use 20 µL per samples)
TLC assay
We analyzed L-aspartate by thin-layer chromatography (TLC). The same amount added Supernatant of synthesis medium to 7mg/mL 5-Dimethylamino naphthalene-1-sulfonyl Chloride (in Acetone) and incubated more than 30min. Two microliters of the sample was placed onto a TLC silica plate, and developed in a mixture of Ethyl acetate, pyridine, water, and acetic acid(162:21:11:6 V/V).
Chemotaxis Assay
Introduction
Chemotaxis by E.coli was assay by some methods. We assay the chemotaxis of E.coli against aspartate using two methods. One was swarming assay. We have used sawaming plate. swarming plate is medium that contained a low concentration by agar. E.coli can swim to aspartate on the swarming plate. The other was capillary assay. We have used capillary that including aspartate. We set capillary in chemotaxis medium including motile E.coli We have determined the number of E.coli attracted to capillary by colony formation on LB plate.
Swarmming Assay
Capillary Assay
Object
To assay chemotaxis by E.coli against aspartarte using capillary.
Plotocol
1. Preculture E.coli with tripton broth at 30℃ 12 hr 50 rpm
2. Check motility under the microscope(×800).
3. Diluting the E.coli culture 100 times. (tripton broth:E.coli culture=20mL : 200μL)
4. Incubate 50rpm 30℃ until log phase (OD600 = ~ 0.2)
5. 500㎕ into micro tubes.
6. Centrifuge (25℃ 10min in 3400G)
7. Dicand the supernatant.
8. Add wash medium(50μL)
9. Resuspending pellets gently.
10. Do 5~9 step repeat.
11. Add chemotaxis medium(500μL)
12. Resuspending pellets gently.
13. Check motility under the microscope(×800).
14. E.coli chemotaxis medium into chamber apparatus.(100μL)
15. Prepare the positive and negative control capillary.(1μL of 10mM aspartate chemotaxis medium and chemotaxis medium into capillary)
16. Two capillary into chamber preparation.
17. 90mim 30℃ incubation.
18. Dilute the chemotaxis buffer in capillary`s liquid 100 times.
19. Prating to LBplate.
20. 37℃ 12h incubation.
21. Counting the colony.
Result & Discussion
TLC Assay

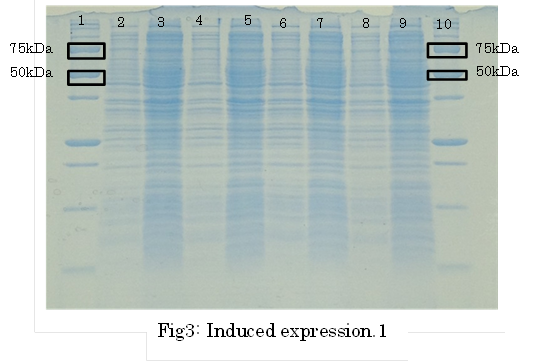
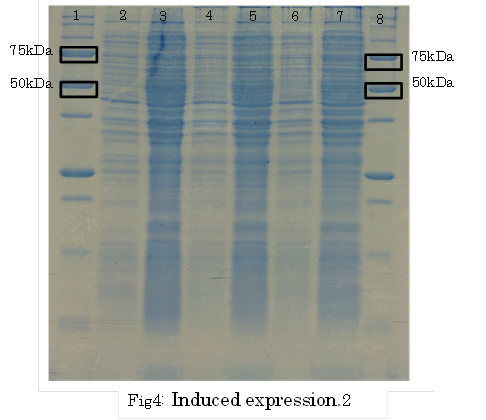
SDS PAGE
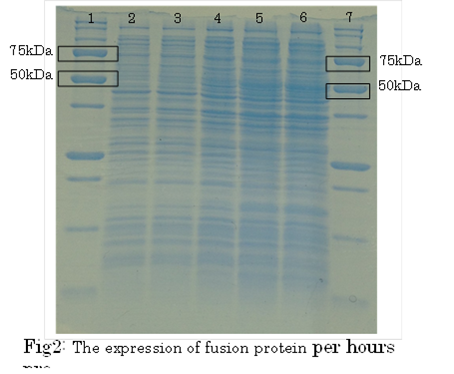 1. marker 2. 0hr 3. 0.5hr 4. 2hr 5. 6hr 6. 24hr 7. Marker
marker:Precision Plus Protein Standards (BIO-RAD)
1. marker 2. 0hr 3. 0.5hr 4. 2hr 5. 6hr 6. 24hr 7. Marker
marker:Precision Plus Protein Standards (BIO-RAD)
OD600=0.74
CdCl2 100μM/IPTG 1mM
Swarming Assay
Capillary Assay
This assay didn`t check chemotaxis by E.coli. We did eleven assays. But all results weren`t authenticity.
 "
"