Team:Freiburg/Content/Results/Vector
From 2014.igem.org
Mirja Harms (Talk | contribs) |
Mirja Harms (Talk | contribs) |
||
| Line 22: | Line 22: | ||
</div> | </div> | ||
</div> | </div> | ||
| - | + | ||
| + | <h2>1.7 Stable integration</h2> | ||
| + | cell line | ||
| + | integration time | ||
| + | |||
| + | <div class="row category-row"> | ||
| + | <div class="col-sm-6"> | ||
| + | <p> | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="col-sm-6"> | ||
| + | <p> | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <figure> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/b/b2/Freiburg2014-10-02_plasmid_map_pMIG_constructs_skizze.JPG"> | ||
| + | <figcaption> | ||
| + | <p class="header">Fig.: pMIG constructs</p> | ||
| + | <p class="desc"></p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | |||
| + | <figure> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/2/20/Freiburg2014-10-02_pMIG_different_consctructs.JPG"> | ||
| + | <figcaption> | ||
| + | <p class="header">Fig.: Transduction efficiency of the viral vector in mouse cells.</p> | ||
| + | <p class="desc"></p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | |||
| + | <blockquote><strong>bla bla</blockquote> | ||
| + | |||
<h2>1.2 High efficiency</h2> | <h2>1.2 High efficiency</h2> | ||
<div class="row category-row"> | <div class="row category-row"> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<p> | <p> | ||
| - | + | The vector we use transfers genes into target genomes. We raised the efficiency in cell targeting and gene transfer to reach an infection rate | |
| - | + | of almost 100% in murine cell lines and high infection rates in other cell lines expressing the murine CAT-1 receptor. Therefore, we optimized | |
| - | production and for | + | protocols for virus |
| + | production and for transducing target cells. Phoenix cells, our producer cell line, were split several times before usage to make sure | ||
to have them healthy and in an exponential growth phase during the production of viral particles. In addition, supernatant containing the vector | to have them healthy and in an exponential growth phase during the production of viral particles. In addition, supernatant containing the vector | ||
were harvest at distinct time points optimized for optimal viral titer. Target cells were infected by viral supernatant with Polybrene that raises | were harvest at distinct time points optimized for optimal viral titer. Target cells were infected by viral supernatant with Polybrene that raises | ||
| Line 35: | Line 70: | ||
were adapted to infection. Last of all, the proportion of viral supernatant to growth medium during infection was optimized to ensure exponential | were adapted to infection. Last of all, the proportion of viral supernatant to growth medium during infection was optimized to ensure exponential | ||
growth and health of target cells. | growth and health of target cells. | ||
| - | Considering all these aspects we | + | Considering all these aspects we reached almost enormous high efficiency in infecting murine cell lines with our viral vector. |
Since we had only 16% efficiency in transferring genes with the vector at the beginning of the projekt, we increased the | Since we had only 16% efficiency in transferring genes with the vector at the beginning of the projekt, we increased the | ||
| Line 61: | Line 96: | ||
<blockquote><strong>With the viral vector used in our system transduction efficiency in murine cells was optimized to almost 100%!</blockquote> | <blockquote><strong>With the viral vector used in our system transduction efficiency in murine cells was optimized to almost 100%!</blockquote> | ||
| - | + | <div class="row category-row"> | |
| - | < | + | <div class="col-sm-6"> |
| - | < | + | <p> |
| - | < | + | </p> |
| + | </div> | ||
| + | |||
| + | <div class="col-sm-6"> | ||
| + | <p> | ||
| + | </p> | ||
| + | <figure> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/8/8a/Freiburg2014-10-02_concentrated_virus_infecting_murine_cells.JPG"> | ||
| + | <figcaption> | ||
| + | <p class="header">Fig.: Concentrated virus</p> | ||
| + | <p class="desc"></p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <blockquote><strong>blubb!</blockquote> | ||
| + | <h2>1.1 Specificity and safety of MuLV</h2> | ||
| + | Half life time of MuLV | ||
| + | |||
<div class="row category-row"> | <div class="row category-row"> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
| Line 118: | Line 172: | ||
<blockquote><strong>The vector derived from the murine leukemia virus is specific for the murine CAT-1 receptor. Therefore | <blockquote><strong>The vector derived from the murine leukemia virus is specific for the murine CAT-1 receptor. Therefore | ||
it is not able to infect human cell lines!</blockquote> | it is not able to infect human cell lines!</blockquote> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 15:11, 3 October 2014
1 The Vector
For stable integration of genes into the genome of target cells we use a vector derived from the murine leukemia virus. The advantages of this vector are its specificity making the viral work safe and easy, its high efficiency for infecting target cells and the ability of stable gene transfer into target genomes. The following results proof these qualities this part of the system.

1.7 Stable integration
cell line integration time

Fig.: pMIG constructs

Fig.: Transduction efficiency of the viral vector in mouse cells.
bla bla
1.2 High efficiency
The vector we use transfers genes into target genomes. We raised the efficiency in cell targeting and gene transfer to reach an infection rate of almost 100% in murine cell lines and high infection rates in other cell lines expressing the murine CAT-1 receptor. Therefore, we optimized protocols for virus production and for transducing target cells. Phoenix cells, our producer cell line, were split several times before usage to make sure to have them healthy and in an exponential growth phase during the production of viral particles. In addition, supernatant containing the vector were harvest at distinct time points optimized for optimal viral titer. Target cells were infected by viral supernatant with Polybrene that raises the probability for particle to reach their target cells. Since murine leukemia viruses only infect dividing cells, growth phases of target cells were adapted to infection. Last of all, the proportion of viral supernatant to growth medium during infection was optimized to ensure exponential growth and health of target cells. Considering all these aspects we reached almost enormous high efficiency in infecting murine cell lines with our viral vector. Since we had only 16% efficiency in transferring genes with the vector at the beginning of the projekt, we increased the
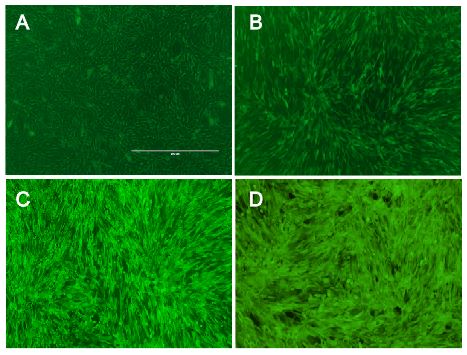
Fig.: nice pictures of nice cells.

Fig.: Transduction efficiency of the viral vector in mouse cells.
With the viral vector used in our system transduction efficiency in murine cells was optimized to almost 100%!

Fig.: Concentrated virus
blubb!
1.1 Specificity and safety of MuLV
Half life time of MuLVAn important aspect for the function of our system as well as for its safety is the specificity of the vector regarding infection of different kind of cells. The vector deriving from the murine leukemia virus is specific for cells carrying the mouse specific CAT-1. Cells that do not have this specific receptor are not targeted by the vector. In order to test the specificity of the system, different kind of cells were incubated with the vector containing EGFP. Since EGFP is stable integrated by the system, infected cells are identified by a green fluorescence that was analysed via flow cytometry (figure 1).
We tested two human cell lines, human embryonic kidney cells as well as human lung epithel carcinoma cells, for their capability of being targeted by the vector. In addition, mouse fibroblasts that express the mouse specific CAT-1 receptor were tested for a positive control. As shown in figure 1 both human cell lines did not express EGFP after incubation with the vector indicating that they were not targeted. However, many cells of the mouse cell line were expressing EGFP after infection.

Fig.1: Description of the experiment (as picture)

Fig.X: FACS analysis of different cell lines incubated with the CAT-1 specific viral vector
Left: Different cell lines not incubated with the vector as negative control, Middle: different cell lines infected with MuLV IRES EGFP (50% in completed growth medium), Right: cells transfected with the mouse specific receptor CAT-1 (rz006) were infected with the viral vector. The infection efficiency is directly correlated with transfection efficiency of the receptor into the different kind of cells.
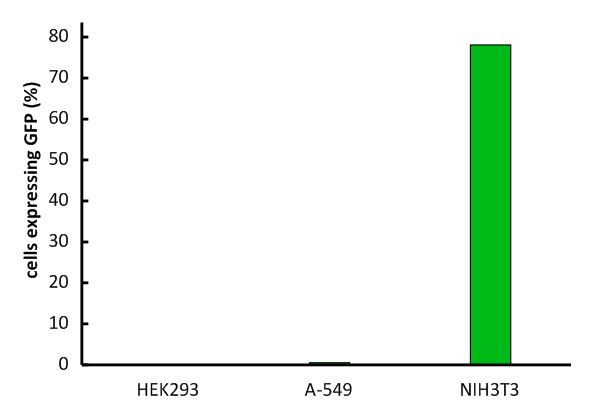
Fig.1: Infection of different cell lines with the viral vector derived from the murine leukemia virus.
In order to investigate the specificity of the viral vector, two human cell lines, human embryonic kidney cells as well as lung epithel carcinoma cells, were infected and the percentage of cells expressing EGFP was analyzed by flow cytometry.
The vector derived from the murine leukemia virus is specific for the murine CAT-1 receptor. Therefore it is not able to infect human cell lines!
 "
"