Team:Paris Saclay/Notebook/September/3
From 2014.igem.org
(→PCR LS) |
(→PCR LS) |
||
| Line 13: | Line 13: | ||
Electrophoresis of the PCR made [https://2014.igem.org/Team:Paris_Saclay/Notebook/September/2#Limonene_synthase_PCR Yesterday] | Electrophoresis of the PCR made [https://2014.igem.org/Team:Paris_Saclay/Notebook/September/2#Limonene_synthase_PCR Yesterday] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
[[File:0309 PCR LS + extraction pPS5.jpg|400px]] | [[File:0309 PCR LS + extraction pPS5.jpg|400px]] | ||
| Line 25: | Line 19: | ||
(well 4-5 = checking of the pps5 Extraction) | (well 4-5 = checking of the pps5 Extraction) | ||
| + | |||
| + | |||
| + | The PCR have success so I use the PCR clean up kit to purify it | ||
| + | |||
| + | Electrophoresis after purification | ||
| + | |||
| + | [[File:0309 purif LS.jpg|400px]] | ||
====Ligation==== | ====Ligation==== | ||
Revision as of 12:00, 7 September 2014
Contents |
LabWork
B- Construction of the fusion protein
We made a classic exttraction of plasmids from the liquid cultures.
Check by electrophoresis if it worked and send it for sequencing.
D- Lemon scent
by Mélanie
PCR LS
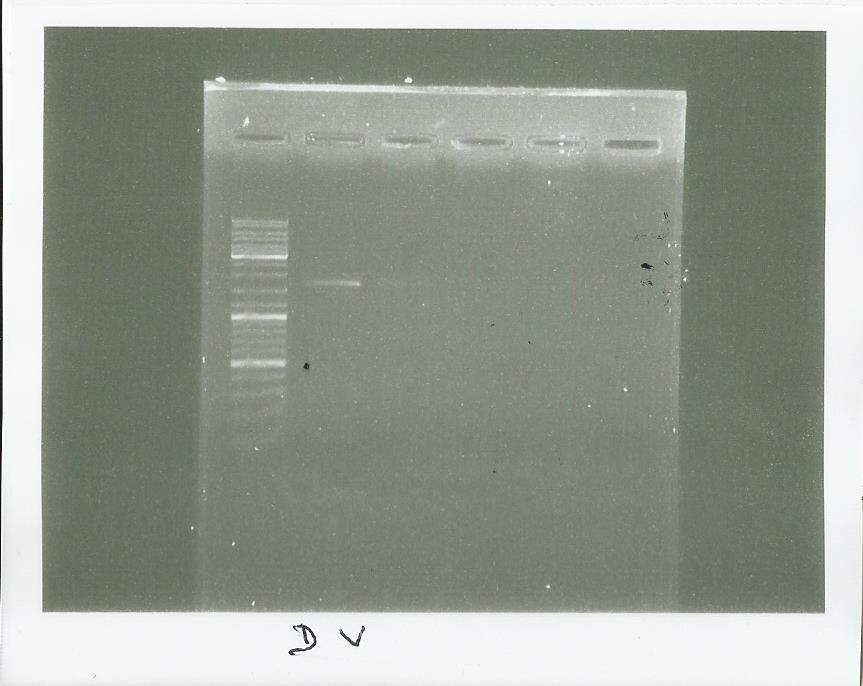
Electrophoresis of the PCR made Yesterday
well 2-3 = PCR with Dream taq or Vent taq
(well 4-5 = checking of the pps5 Extraction)
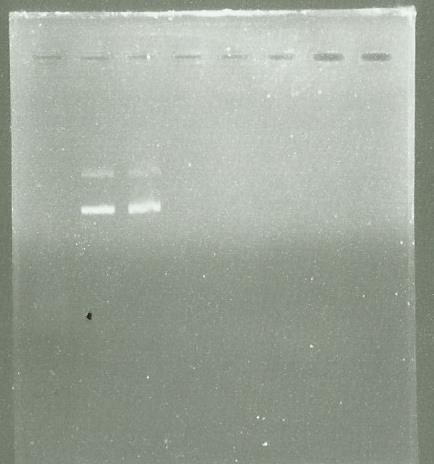
The PCR have success so I use the PCR clean up kit to purify it
Electrophoresis after purification
Ligation
We already have some pPSI digested and dephosphorelated
So I do a ligation:
| component | volume |
|---|---|
| H2O | 5μl |
| buffer | 2μl |
| ligase | 1μl |
| pPSI | 2μl |
| LS PCR | 10μl |
2 hours at room temperature and over night at 4°
pPS5
Plasmid exctraction using the kit (picture is here)
digestion by SalI
| component | volume |
|---|---|
| H2O | 11μl |
| buffer | 5μl |
| SalI | 2μl |
| pPS5 | 30μl |
Electrophoresis
We saw that we have something strang with our plasmid but we wil check it tomorrow with a good ladder (the ladder here was normally on the well 1)
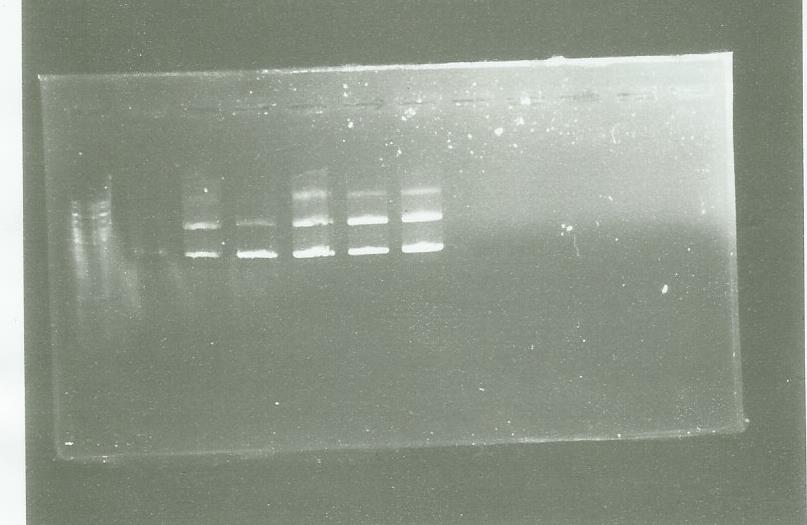
pPS3 and pPS4
We digest the plasmid by HindIII to direct our insert
| component | volume |
|---|---|
| H2O | 6μl |
| buffer | 1μl |
| HindIII | 1μl |
| pPS3/4 | 2μl |
well 1 = ladder well 2-3-4 = pPS3 Well 5-6-7 = pPS4
results are very strange
 "
"