Team:Oxford/InterlabDevices
From 2014.igem.org
| Line 22: | Line 22: | ||
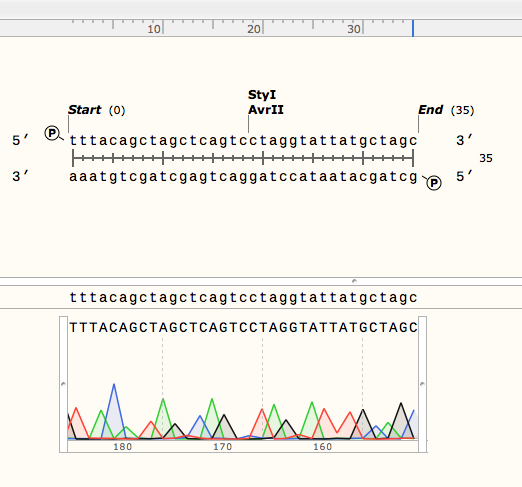
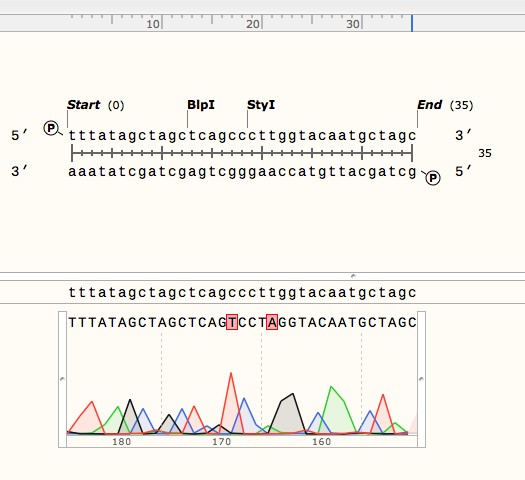
<p> We received sequencing results that confirmed the sequences of the parts we were using from the DNA-distribution kit, including the two point-mutations in part 3A. Shown below are the sequence alignments of the sequence we expected (top strand) and the sequencing results (bottom) of parts 2A (left) and 3A (right):</p> | <p> We received sequencing results that confirmed the sequences of the parts we were using from the DNA-distribution kit, including the two point-mutations in part 3A. Shown below are the sequence alignments of the sequence we expected (top strand) and the sequencing results (bottom) of parts 2A (left) and 3A (right):</p> | ||
</html> | </html> | ||
| - | [[File:Sequence_Alignment_BBa_J23101.png|left| | + | [[File:Sequence_Alignment_BBa_J23101.png|left|450px]][[File:Sequence_Alignment_BBa_J23115.png|right|450px]] |
<html> | <html> | ||
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
Revision as of 14:22, 1 August 2014
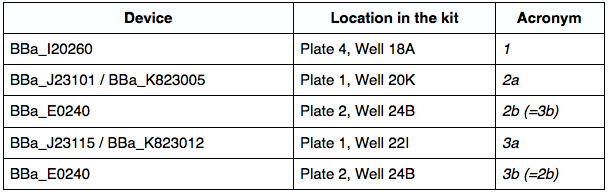
Devices
I. Using the DNA distribution kit to extract devices 1-3:
We filled the 4 wells we needed with 10 uL of dH2O and let it sit for about 10 minutes to resuspend. We transformed the resuspended DNA into chemically competent E.coli cells (DH5) and plated them on an agar plate with kanamycin (plate for device 1) and chloramphenicol (plates for devices 2a, 2b/3b, and 3a) using the standard E.coli transformation protocol (however, we used 200 uL of competent cells per transformation rather than 100uL).
II. Growing liquid cultures:
The next day, we have collected our plates from the 37°C incubator. All plates had colonies growing on them and we took one of each for generating liquid cultures.
III. MiniPrep and Sending for Sequencing:
Having let the cultures grow up overnight, we took samples from each culture and used the Miniprep kits to extract each plasmid. Using the Nanodrop to measure the DNA concentration we prepared them for sequencing by SourceBioscience. The 5uL sequencing samples were frozen overnight and then sent for sequencing the next day. The remaining 45uL were stored in the -20⁰c freezer.
IV. Sequencing results:
We received sequencing results that confirmed the sequences of the parts we were using from the DNA-distribution kit, including the two point-mutations in part 3A. Shown below are the sequence alignments of the sequence we expected (top strand) and the sequencing results (bottom) of parts 2A (left) and 3A (right):
V. Restriction digest:
 "
"