Team:Austin Texas/photocage
From 2014.igem.org
Nathanshin (Talk | contribs) |
|||
| Line 97: | Line 97: | ||
=Background= | =Background= | ||
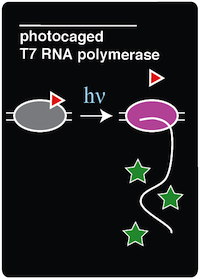
| - | Tyrosine residue 639 (Y639) was specifically targeted because it lies on a crucial position on the O-helix domain of T7 RNAP and has been proven to be essential for polymerization. ( | + | Tyrosine residue 639 (Y639) was specifically targeted because it lies on a crucial position on the O-helix domain of T7 RNAP and has been proven to be essential for polymerization. (Chou et al. 2010) The Y639 residue in the O-helix is responsible for two major roles in the elongation stage of DNA polymerization. First, this tyrosine residue discriminates between deoxyribose and ribose substrates using the Tyrosyl-OH (Temiakov et al. 2004). Second, Y639 is responsible for moving newly synthesized RNA out of the catalytic site and preparing for the next NTP to be inserted (Achenbach 2004). These functions of the O-helix were shown to be essential through mutational analysis (Osumidavis 1994). Introducing a bulky group such as ONBY in place of tyrosine renders the enzyme nonfunctional in several ways. First, the native tyrosine-OH is not there anymore to coordinate Mg2+, which plays an essential role in discriminating between deoxyribose and ribose substrates. Additionally, because of the sterics of the ONBY molecule itself, it blocks incoming nucleotides from entering the active site. Because the loss of this tyrosine residue in the active site leads to a non-functional polymerase, Y639 proved to be a good candidate for incorporating a photocaged amino acid (Chou et al. 2010). |
[[Image:Uncaging_of_ONBY.jpg | 300px|left|thumb| Figure 2. The caged T7 RNAP is decaged via exposure to 365 nm light. '''Chou et al. 2010''']] | [[Image:Uncaging_of_ONBY.jpg | 300px|left|thumb| Figure 2. The caged T7 RNAP is decaged via exposure to 365 nm light. '''Chou et al. 2010''']] | ||
| - | Incorporation of ONBY at position 639 of T7 RNAP halts activity because of the bulky nature of ONBY ( | + | Incorporation of ONBY at position 639 of T7 RNAP halts activity because of the bulky nature of ONBY (Chou et al. 2010). This ONB side group effectively renders T7 RNAP inactive. However, the bulky ONB group is able to be removed through irradiation with 365 nm light. The wavelength of light used to "decage" the amino acid proved to be another advantage of this system because 365 nm light is not toxic to the cell (Chou et al 2010). Once the ONB group is removed, a normal tyrosine residue is left in its place, restoring T7 RNA polymerase activity. |
| - | In order to incorporate the ncAA into amberless E.coli (which is described '''[here]'''), a ''Methanocaldoccus jannaschii'' tyrosyl-tRNA synthetase/tRNA pair was previously mutated to selectively charge and incorporate ONBY. Six residues (Tyr 32, Leu 65, Phe 108, Gln 109, Asp 158, and Leu 162) on the original synthetase were randomized and the library was selected for its ability to charge ONBY while discriminating against other canonical amino acids. The resulting mutant ONBY synthetase contained five mutations ( | + | In order to incorporate the ncAA into amberless E.coli (which is described '''[here]'''), a ''Methanocaldoccus jannaschii'' tyrosyl-tRNA synthetase/tRNA pair was previously mutated to selectively charge and incorporate ONBY. Six residues (Tyr 32, Leu 65, Phe 108, Gln 109, Asp 158, and Leu 162) on the original synthetase were randomized and the library was selected for its ability to charge ONBY while discriminating against other canonical amino acids. The resulting mutant ONBY synthetase contained five mutations (Deiters et al. 2006). The following are the residues that were mutated on the synthetase: Tyr32→Gly32, Leu65→Gly65, Phe108→Glu108, Asp158→Ser158, and Leu162→Glu162. The Asp158→Ser158 and Tyr32→Gly32 mutations are believed to result in the loss of hydrogen bonds with the natural substrate, which would disfavor binding to tyrosine. Additionally, the Tyr 32→Gly 32 and Leu 65→Gly 65 mutations are believed to increase the size of the substrate-binding pocket to accommodate the bulky o-nitrobenzyl group (Deiters et al. 2006). |
| - | + | ||
=Experimental Methods= | =Experimental Methods= | ||
| Line 169: | Line 168: | ||
*P. A. Osumidavis, N. Sreerama, D. B. Volkin, C. R. Middaugh, R. W. Woody, A. Y. M. Woody, J. Mol. Biol. 1994, 237, 5 – 19. | *P. A. Osumidavis, N. Sreerama, D. B. Volkin, C. R. Middaugh, R. W. Woody, A. Y. M. Woody, J. Mol. Biol. 1994, 237, 5 – 19. | ||
*D. Temiakov, V. Patlan, M. Anikin, W. T. McAllister, S. Yokoyama, D. G. Vassylyev, Cell 2004, 116, 381 –391. | *D. Temiakov, V. Patlan, M. Anikin, W. T. McAllister, S. Yokoyama, D. G. Vassylyev, Cell 2004, 116, 381 –391. | ||
| - | + | *J. C. Achenbach, W. Chiuman, R. P. G. Cruz, Y. Li, Curr. Pharm. Biotechnol. 2004, 5, 321 –336. | |
| - | + | *P. A. Osumidavis, N. Sreerama, D. B. Volkin, C. R. Middaugh, R. W. Woody, A. Y. M. Woody, J. Mol. Biol. 1994, 237, 5 – 19 | |
Revision as of 00:33, 17 October 2014
| |||||||||||||||||||||||||||||
 "
"