Team:Austin Texas/kit
From 2014.igem.org
(→Experimental Preparation) |
(→Experimental Preparation) |
||
| Line 146: | Line 146: | ||
==Experimental Preparation== | ==Experimental Preparation== | ||
| + | |||
| + | |||
| + | [[File:Alex and Foil-covered incubator.jpg|200px|thumb|right|Protection of light-sensitive ncAAs by using a foil-wrapped incubator.]] | ||
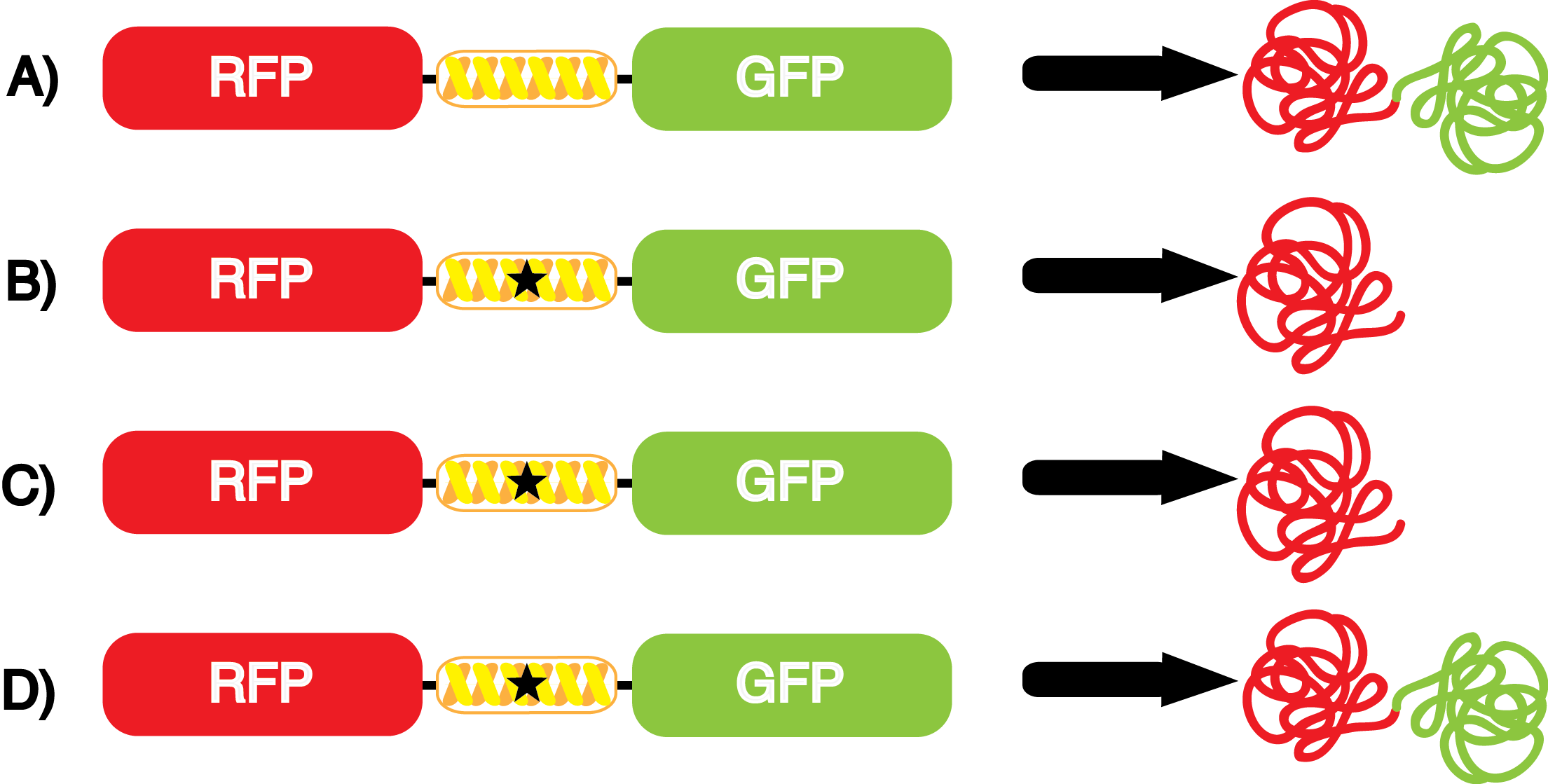
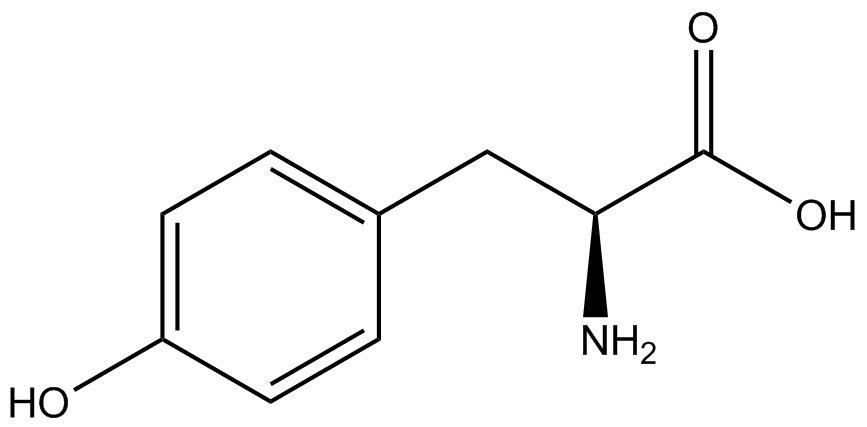
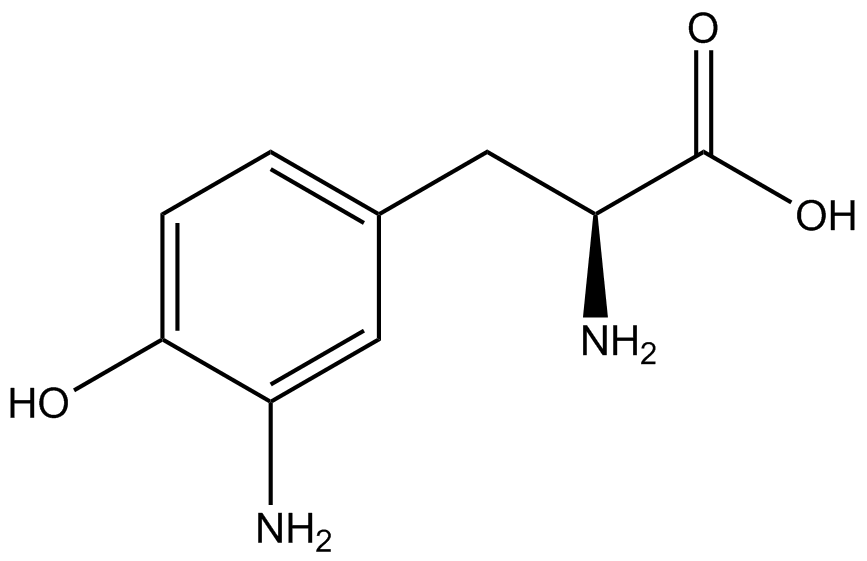
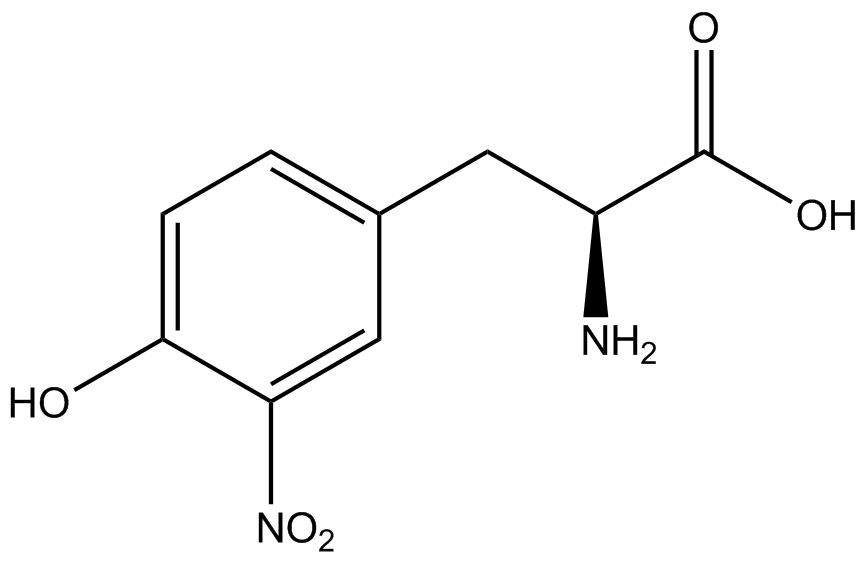
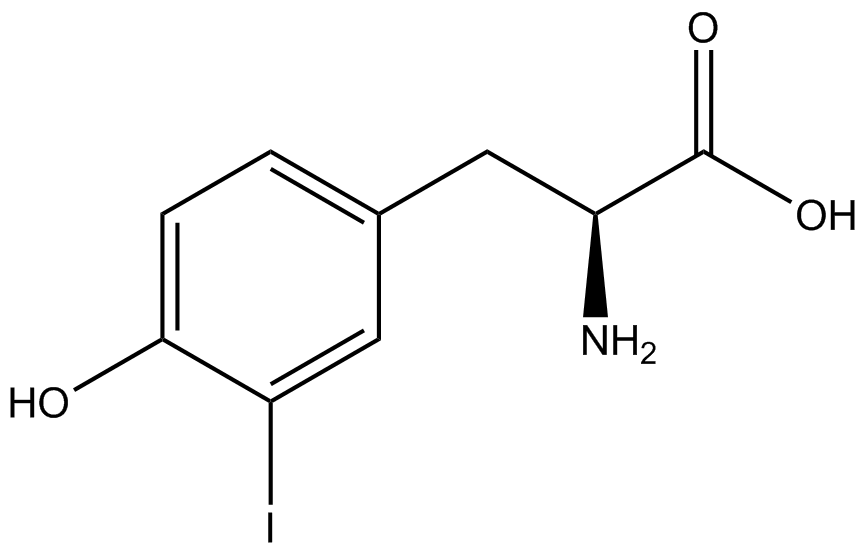
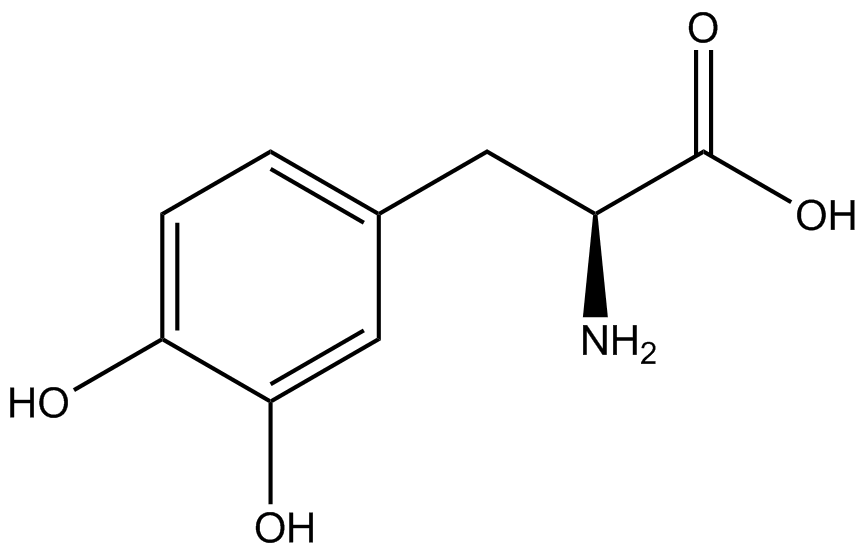
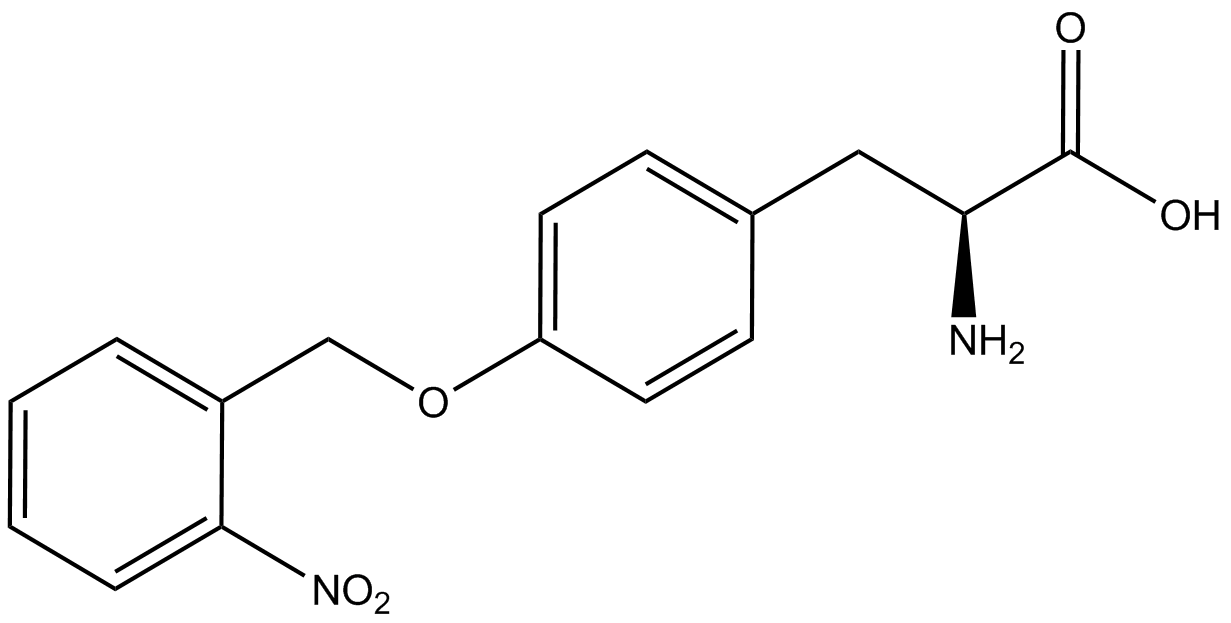
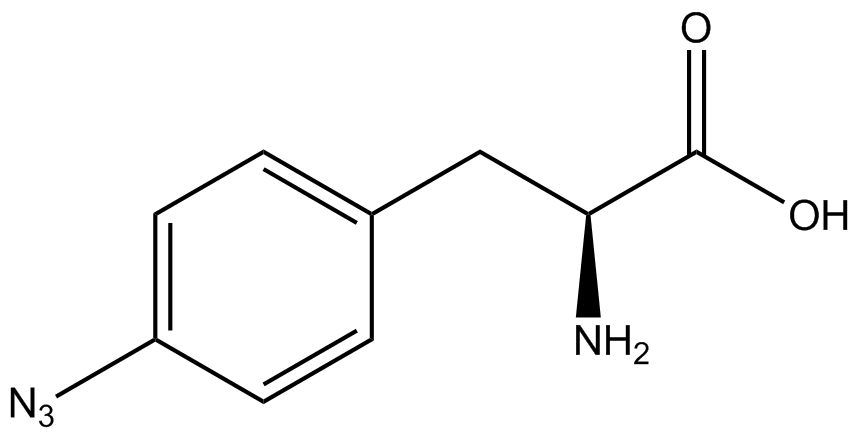
An ncAA synthetase/tRNA pair was cloned into pStG and transformed into pFRYC amberless ''E. coli''. and pFRY amberless ''E. coli''. Other necessary control strains include RFP amberless ''E. coli'' (RFP control), sfGFP amberless ''E. coli'' (GFP control), amberless ''E. coli'' (cell background control), and LB media supplemented with ncAA (media background control). An overnight culture of each strain was grown in LB with the appropriate antibiotics at 37ºC and 225rpm. 10 mL of media with the appropriate antibiotics was inoculated with 100 µL of overnight culture and allowed to grow in the same conditions until the culture density was ~0.2-0.3 OD, or ~3 hours. The 10 mL culture was split between 4 different sterile test-tubes, 2 mL of culture per tube. The conditions of the four test tubes were as follows: | An ncAA synthetase/tRNA pair was cloned into pStG and transformed into pFRYC amberless ''E. coli''. and pFRY amberless ''E. coli''. Other necessary control strains include RFP amberless ''E. coli'' (RFP control), sfGFP amberless ''E. coli'' (GFP control), amberless ''E. coli'' (cell background control), and LB media supplemented with ncAA (media background control). An overnight culture of each strain was grown in LB with the appropriate antibiotics at 37ºC and 225rpm. 10 mL of media with the appropriate antibiotics was inoculated with 100 µL of overnight culture and allowed to grow in the same conditions until the culture density was ~0.2-0.3 OD, or ~3 hours. The 10 mL culture was split between 4 different sterile test-tubes, 2 mL of culture per tube. The conditions of the four test tubes were as follows: | ||
| Line 154: | Line 157: | ||
IPTG stock solution was made at 1000X concentration ('''why is this here?''') and the ncAA was added to yield a concentration of 1 mM. Sterile deionized water was added in the place of ncAA and IPTG as a control ('''?'''). Once the controls, IPTG, and the ncAA were added appropriately, the cultures were allowed to grow to ~0.5 OD. 70 µL of each culture condition and control culture was added to a separate wells in a transparent 96-well plate for fluorescence and OD readings in a microplate reader. | IPTG stock solution was made at 1000X concentration ('''why is this here?''') and the ncAA was added to yield a concentration of 1 mM. Sterile deionized water was added in the place of ncAA and IPTG as a control ('''?'''). Once the controls, IPTG, and the ncAA were added appropriately, the cultures were allowed to grow to ~0.5 OD. 70 µL of each culture condition and control culture was added to a separate wells in a transparent 96-well plate for fluorescence and OD readings in a microplate reader. | ||
| + | |||
| + | |||
| + | |||
| + | |||
[[File:10-14-14 big experiment test tubes.png|450px|thumb|left| Preparation for the ncAA Kit Test]] | [[File:10-14-14 big experiment test tubes.png|450px|thumb|left| Preparation for the ncAA Kit Test]] | ||
[[File:RFP-GFP Controls 10-14-15.png|200px|thumb|right|(+/-) IPTG]] | [[File:RFP-GFP Controls 10-14-15.png|200px|thumb|right|(+/-) IPTG]] | ||
| + | |||
Revision as of 13:48, 15 October 2014
|
 "
"