Team:Austin Texas/photocage
From 2014.igem.org
Nathanshin (Talk | contribs) (→Background) |
Nathanshin (Talk | contribs) (→Background) |
||
| Line 85: | Line 85: | ||
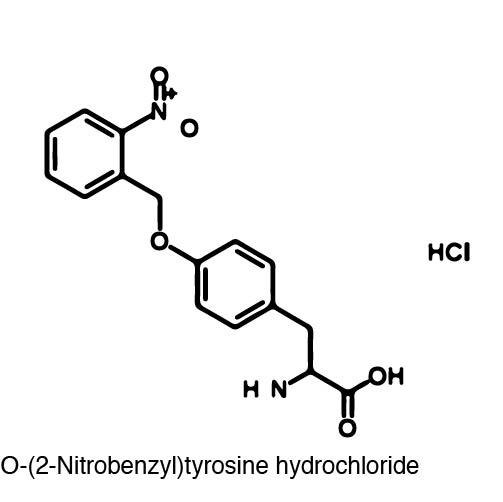
Because of the bulky nature of ONBY, its incorporation at position 639 essentially halted the activity of the T7 polymerase. However, the bulky ONB group is able to be removed through irradiation with 365 nm light. The wavelength of light used to "decage" the amino acid proved to be another advantage because 365 nm light is not toxic to the cell. Once the ONB group is removed, a normal tyrosine residue is left which restores RNA polymerase activity. | Because of the bulky nature of ONBY, its incorporation at position 639 essentially halted the activity of the T7 polymerase. However, the bulky ONB group is able to be removed through irradiation with 365 nm light. The wavelength of light used to "decage" the amino acid proved to be another advantage because 365 nm light is not toxic to the cell. Once the ONB group is removed, a normal tyrosine residue is left which restores RNA polymerase activity. | ||
| - | In order to incorporate the non-canonical amino acid into E.coli, a Methanococcus jannaschii synthetase/tRNA pair had to be mutated to selectively charge and incorporate ONBY. Six residues (Tyr 32, Leu 65, Phe 108, Gln 109, Asp 158, and Leu 162) on the original synthetase were randomized and selected for its ability to charge ONBY while discriminating against other canonical amino acids. The Asp 158→Ser 158 and Tyr 32→Gly 32 mutations were likely involved in the loss of hydrogen bonds with the natural substrate, which would disfavor binding to natural tyrosine. On the other hand, the Tyr 32→Gly 32 and Leu 65→Gly 65 mutations likely increase the size of the substrate-binding pocket to accommodate the bulky o-nitrobenzyl group. | + | In order to incorporate the non-canonical amino acid into the amberless E.coli (which is described [here]), a Methanococcus jannaschii synthetase/tRNA pair had to be mutated to selectively charge and incorporate ONBY. Six residues (Tyr 32, Leu 65, Phe 108, Gln 109, Asp 158, and Leu 162) on the original synthetase were randomized and selected for its ability to charge ONBY while discriminating against other canonical amino acids. The Asp 158→Ser 158 and Tyr 32→Gly 32 mutations were likely involved in the loss of hydrogen bonds with the natural substrate, which would disfavor binding to natural tyrosine. On the other hand, the Tyr 32→Gly 32 and Leu 65→Gly 65 mutations likely increase the size of the substrate-binding pocket to accommodate the bulky o-nitrobenzyl group. |
Revision as of 21:24, 11 October 2014
|
 "
"