Team:SUSTC-Shenzhen/Notebook/HeLaCell/Construct-Cellline
From 2014.igem.org
m (minor improve) |
|||
| Line 13: | Line 13: | ||
date=2014/8/15| | date=2014/8/15| | ||
goal=Construct stably transfected HeLa cells!}} | goal=Construct stably transfected HeLa cells!}} | ||
| - | |||
| - | |||
| Line 33: | Line 31: | ||
==Cell transfection== | ==Cell transfection== | ||
| - | [2014 Aug 15th | + | |
| + | [2014 Aug 15th 11:00am] | ||
===Materials=== | ===Materials=== | ||
| Line 60: | Line 59: | ||
|} | |} | ||
<html></div></html> | <html></div></html> | ||
| + | |||
===Method=== | ===Method=== | ||
| Line 126: | Line 126: | ||
{{SUSTC-Image|wiki/images/2/26/SUSTC-Shenzhen-Lipo3000_protocol.png|Lipo-protocol}} | {{SUSTC-Image|wiki/images/2/26/SUSTC-Shenzhen-Lipo3000_protocol.png|Lipo-protocol}} | ||
The official protocol of Lipofectamine 3000 (Invitrogen) | The official protocol of Lipofectamine 3000 (Invitrogen) | ||
| - | We use the reduced amout of DNA. | + | We use the reduced amout of Lipofectamine(lipo), and slightly improve the procedure by the experience of an RA. |
| + | # '''Dilute 3.75μl/well Lipofectamine 3000 reagent in 25μl/well Opti-MEM, and incubation for 5min''' | ||
| + | # Prepare master mix of 5DNA μg/well by dilutiong DNA in Opti-MEM medium 25μl/well according to the form, then add P3000 reagent. | ||
| + | # Add dilutetd DNA to each tube of diluted lipo 3000. | ||
| + | # '''Incubation for 10min ''' | ||
| + | # Add DNA lipid complex to cell waiting for the harvest. | ||
| + | |||
| + | ====Laboratory note==== | ||
| + | |||
| + | # Add DNA to 380μL Opti-MEM medium according to the table 2 and add 15μL P3000 reagent, mix well. | ||
| + | # Dilute Lipofectamine 3000 Reagent in Opti-MEM Medium: (2x) 1000μL Opti-MEM Medium + 120μL Lipofectamine 3000 Reagent, mix well. | ||
| + | # Add 395μL Diluted Lipofectamine 3000 to Diluted DNA of each group (1:1 ratio), mix well. | ||
| + | # Incubate for about 10min. | ||
| + | # Add DNA-lipid complex (250μL per well) to cells, shake the plate. (12:00 am) | ||
| + | # After 10h, change the medium with the complete medium with 10% FBS and Penicillin-Streptomycin. | ||
| + | |||
| + | ==Fluorescent Observation== | ||
| + | |||
| + | [2014 Aug 16th 9:00am] Change the medium of transfected cells and observe the fluorescent with fluorescent microscope. | ||
| + | |||
| + | ===Laboratory note==== | ||
| + | |||
| + | The transfected cells are observed before we change the medium. | ||
| + | //TODO figure | ||
| + | |||
| + | |||
| + | |||
| + | It is found that part of the cells round and suspended, and part of cells just become round. But the morphology of most of the cells are normal. | ||
| + | |||
| + | //TODO figure | ||
| + | ==Passage cell to dish== | ||
| + | [2014 Aug 16th 15:00pm] | ||
| + | Passage part of cells to the 10cm petri dish (for picking monoclonal later except G5), and the remaining cells are still seeded on the previous 24-well plates (about 500μL cell suspension per well). (15:00 pm – 18:00 pm) | ||
| + | For every group, two dishes with 5,000 cells seeded and two dished with 50,000 cells seeded. | ||
| + | |||
| + | ===Procedures=== | ||
| + | # Wash cells with PBS, trypsin digestion, add medium to stop digestion | ||
| + | # Transfer the cell suspension into 15 mL centrifuge tube and centrifuge at 800rpm for 5 min. | ||
| + | # Discard the supernatant and add 2 mL complete medium to suspend the cells. | ||
| + | # Count the cell concentration with blood counting chamber. | ||
| + | # Mark the new dishes, add 5 mL medium to every dish, and add corresponding cell suspension, mix well with the pipet. | ||
| + | # Culture. | ||
| + | <html><div class="table-responsive"></html> | ||
| + | {|class="table" | ||
| + | |+Concentration of the cells | ||
| + | !Group | ||
| + | !Counted number | ||
| + | !Concentration (cells/mL) | ||
| + | !Volume needed | ||
| + | |- | ||
| + | |1 | ||
| + | |30 | ||
| + | |300,000 | ||
| + | |167 and 16.7 | ||
| + | |- | ||
| + | |2 | ||
| + | |20 | ||
| + | |200,000 | ||
| + | |294 and 29.4 | ||
| + | |- | ||
| + | |3 | ||
| + | |30 | ||
| + | |300,000 | ||
| + | |294 and 29.4 | ||
| + | |- | ||
| + | |4 | ||
| + | |30 | ||
| + | |300,000 | ||
| + | |143 and 14.3 | ||
| + | |} | ||
| + | <html></div></html> | ||
| + | //TODO{ | ||
| + | ===Discussion=== | ||
| + | |||
| + | When counting cells, some dead cells were observed, and those cells were not included. The second day morning, some of the cells on the petri dish are round and suspended. Cells were few and scattered. For dishes with 5,000 cells, only several cells can be observed in one field under 4X objective lens. For dishes with 50,000 cells, near 10-20 cells can be observed in one field under 4X objective lens. | ||
| + | |||
| + | ==Fluorescent observation== | ||
| + | [2014 Aug 18th 10:00am] | ||
| + | Observe the transfected cells on the plate under fluorescence microscope | ||
| + | //TODO figure | ||
| + | |||
| + | ==Cell selection== | ||
| + | [2014 Aug 18th 10:00am] | ||
| + | |||
| + | |||
| + | //TODO} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 15:14, 11 October 2014
Notebook
Elements of the endeavor.
Contents |
Stably Transfected HeLa cell line construct
2014/8/15 Construct stably transfected HeLa cells!
Seed cells
[2014 Aug 14th 15:00pm]
Method
Seed HeLa cells in 6-well-plate (15wells at total), 200,000 cells per well. For tansfection
- Procedure
- Wash cells with PBS, trypsin digestion, and medium to stop digestion.
- Transfer the sell suspension in 15ml centrifuge tube and centrifuge at 800rpm, fro 5 min.
- Discard the supernatant and add 2ml complete medium to re suspend the cells.
- Add 1ml PBS to every gap between well
- 1:10 dilution count the cell concentration with blood counting slide. Get the concentration: 2,800,000 cells/ml. 1.15ml cell suspendion + about 31ml DMEM with 5% FBS. Mix well and add 2ml per well, shake the plate gently.
Result
The cells plate unevenly concentrated at the center of each well.
Cell transfection
[2014 Aug 15th 11:00am]
Materials
- plasmid pBx-083 (EGFP)
- plasmid Piggybac
- plasmid Cas9+TetOn
- Lipofectamine 3000(Invitrogen)
- Opti-MEM
- HeLa cells in 6-well-plate
| Plasmid | Concentration(ng/μl) |
|---|---|
| EGFP(pBx-083) | 1641 |
| Cas9+TetOn | 2344.1 |
| Piggybac | 2896.7 |
Method
a. 3 combinations
- EGFP(pBx-083) & Piggybac(transposase)
- EGFP & Cas9 & Piggybac
- Cas9(Cas9+TetOn) & Piggybac
For the first two combination we have different ratio of EGFP to get mono-copy of EGFP in cell line.
| Group 1 | EGFP:Piggybac = 1:1 |
|---|---|
| Group 2 | EGFP:Piggybac = 1:9 |
| Group 3 | Cas9:EGFP:Piggybac = 1:1:1 |
| Group 4 | Cas9:EGFP:Piggybac = 3:17:10 |
| Group 5 | Cas9:Piggybac = 1:1 |
| Groups(3wells/group) | EGFP(μl) | Cas9(μl) | Piggybac(μl) |
|---|---|---|---|
| 2.29 | 0 | 1.30 | |
| 0.46 | 0 | 2.33 | |
| 1.49 | 1.359 | 0.612 | |
| 0.46 | 1.81 | 0.86 | |
| 0 | 1.60 | 1.30 |
Procedure
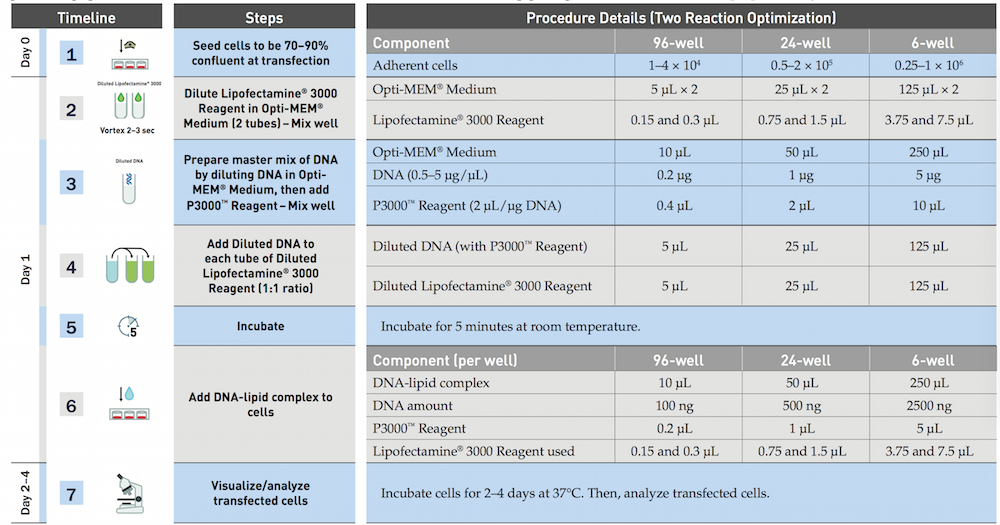
The official protocol of Lipofectamine 3000 (Invitrogen)
We use the reduced amout of Lipofectamine(lipo), and slightly improve the procedure by the experience of an RA.
- Dilute 3.75μl/well Lipofectamine 3000 reagent in 25μl/well Opti-MEM, and incubation for 5min
- Prepare master mix of 5DNA μg/well by dilutiong DNA in Opti-MEM medium 25μl/well according to the form, then add P3000 reagent.
- Add dilutetd DNA to each tube of diluted lipo 3000.
- Incubation for 10min
- Add DNA lipid complex to cell waiting for the harvest.
Laboratory note
- Add DNA to 380μL Opti-MEM medium according to the table 2 and add 15μL P3000 reagent, mix well.
- Dilute Lipofectamine 3000 Reagent in Opti-MEM Medium: (2x) 1000μL Opti-MEM Medium + 120μL Lipofectamine 3000 Reagent, mix well.
- Add 395μL Diluted Lipofectamine 3000 to Diluted DNA of each group (1:1 ratio), mix well.
- Incubate for about 10min.
- Add DNA-lipid complex (250μL per well) to cells, shake the plate. (12:00 am)
- After 10h, change the medium with the complete medium with 10% FBS and Penicillin-Streptomycin.
Fluorescent Observation
[2014 Aug 16th 9:00am] Change the medium of transfected cells and observe the fluorescent with fluorescent microscope.
Laboratory note=
The transfected cells are observed before we change the medium. //TODO figure
It is found that part of the cells round and suspended, and part of cells just become round. But the morphology of most of the cells are normal.
//TODO figure
Passage cell to dish
[2014 Aug 16th 15:00pm] Passage part of cells to the 10cm petri dish (for picking monoclonal later except G5), and the remaining cells are still seeded on the previous 24-well plates (about 500μL cell suspension per well). (15:00 pm – 18:00 pm) For every group, two dishes with 5,000 cells seeded and two dished with 50,000 cells seeded.
Procedures
- Wash cells with PBS, trypsin digestion, add medium to stop digestion
- Transfer the cell suspension into 15 mL centrifuge tube and centrifuge at 800rpm for 5 min.
- Discard the supernatant and add 2 mL complete medium to suspend the cells.
- Count the cell concentration with blood counting chamber.
- Mark the new dishes, add 5 mL medium to every dish, and add corresponding cell suspension, mix well with the pipet.
- Culture.
| Group | Counted number | Concentration (cells/mL) | Volume needed |
|---|---|---|---|
| 1 | 30 | 300,000 | 167 and 16.7 |
| 2 | 20 | 200,000 | 294 and 29.4 |
| 3 | 30 | 300,000 | 294 and 29.4 |
| 4 | 30 | 300,000 | 143 and 14.3 |
Discussion
When counting cells, some dead cells were observed, and those cells were not included. The second day morning, some of the cells on the petri dish are round and suspended. Cells were few and scattered. For dishes with 5,000 cells, only several cells can be observed in one field under 4X objective lens. For dishes with 50,000 cells, near 10-20 cells can be observed in one field under 4X objective lens.
Fluorescent observation
[2014 Aug 18th 10:00am] Observe the transfected cells on the plate under fluorescence microscope //TODO figure
Cell selection
[2014 Aug 18th 10:00am]
//TODO}
 "
"