Team:Tuebingen/Project/Enzymes
From 2014.igem.org
Patrick 1990 (Talk | contribs) |
|||
| (8 intermediate revisions not shown) | |||
| Line 17: | Line 17: | ||
<p> | <p> | ||
| - | The α-<i>N</i>-acetylgalactosaminidase (NAGA) from <i>Elizabethkingia meningoseptica</i> is an enzyme which is capable of cleaving off the <i>N</i>-acetylgalactosamine residue of the A antigen. This enzyme consists of 448 amino acids and weights about 50.5 kDa. The structure is determined and can be found in the <a href= | + | The α-<i>N</i>-acetylgalactosaminidase (NAGA) from <i>Elizabethkingia meningoseptica</i> is an enzyme which is capable of cleaving off the <i>N</i>-acetylgalactosamine residue of the A antigen. This enzyme consists of 448 amino acids and weights about 50.5 kDa. The structure is determined and can be found in the <a href="http://www.rcsb.org/pdb/explore.do?structureId=2ixa">RCSB Protein Data Base</a>. |
| - | The NAGA uses an unusual mechanism to achieve its reaction. A catalytic NAD<sup>+</sup> accepts a hydride ion from C<sub>3</sub> of the <i>N</i>-acetylgalactosamine which leads to formation of an carbonyl group at this position. The carbonyl group at C<sub>3</sub> enables the abstraction of a proton at C<sub>2</sub> by stabilizing the temporary negative charge. The negative charge leads to the possibility to release the residual carbohydrates as an alcoholate and replacing them with an hydroxide ion. The catalytic cycle is finished by the regeneration of the NAD<sup>+</sup> through reduction of the carbonyl function at C<sub>3</sub>. </p> | + | |
| + | </p><img src="https://static.igem.org/mediawiki/2014/8/89/Tue2014_12IXA.jpg"style="width: 400px;"> | ||
| + | <p id="picTextCenter">Figure 1: α-<i>N</i>-acetylgalactosaminidase (2IXA) from <i>Elizabethkingia meningoseptica</i> </p> | ||
| + | |||
| + | The NAGA uses an unusual mechanism to achieve its reaction. A catalytic NAD<sup>+</sup> accepts a hydride ion from C<sub>3</sub> of the <i>N</i>-acetylgalactosamine which leads to formation of an carbonyl group at this position. The carbonyl group at C<sub>3</sub> enables the abstraction of a proton at C<sub>2</sub> by stabilizing the temporary negative charge. The negative charge leads to the possibility to release the residual carbohydrates as an alcoholate and replacing them with an hydroxide ion. The catalytic cycle is finished by the regeneration of the NAD<sup>+</sup> through reduction of the carbonyl function at C<sub>3</sub>. | ||
| + | </p><img src="https://static.igem.org/mediawiki/2014/6/63/Tuebingen-NAGAMechanism1.jpg"style="width: 400px;"> | ||
| + | <p id="picTextCenter">Figure 2: Mechanism of the NAGA according to Liu et al.</p> | ||
| + | </p><img src="https://static.igem.org/mediawiki/2014/b/be/Tuebingen-NAGA-Mechanism2.jpg"style="width: 400px;"> | ||
| + | <p id="picTextCenter">Figure 3: Oxidation at C<sub>3</sub> improves the ability to deprotonate C<sub>2</sub></p> | ||
<p> | <p> | ||
| - | α-Galactosidase (aGal) from <i>Bacteroides fragilis</i> is able to cleave off the terminal galactose residue of the B antigen. The enzyme is 589 amino acids long and weighs 66.12 kDa. The structure of the enzyme is not solved up to today. Kinetic properties were determined in the publication of <a href= | + | α-Galactosidase (aGal) from <i>Bacteroides fragilis</i> is able to cleave off the terminal galactose residue of the B antigen. The enzyme is 589 amino acids long and weighs 66.12 kDa. The structure of the enzyme is not solved up to today. Kinetic properties were determined in the publication of <a href="http://www.nature.com/nbt/journal/v25/n4/abs/nbt1298.html">Liu et al. (2007)</a>. |
</p> | </p> | ||
| + | <p> | ||
| + | The <a href="http://pubs.acs.org/doi/abs/10.1021/bi900991h">endo-β-galactosidase (EABase)</a> from <i>Clostridium perfringens</i> is able to accept the A and the B antigen as substrate and attacks the bond between galactose and <i>N</i>-acetylglucosamine. As a consequence, EABase releases the blood group trisaccharides and generates a precursor of the blood antigen H. The enzyme is 804 amino acids long and weights 91.25 kDa. The structure of the binding module of this enzyme is determined and can be accessed in the <a href="http://www.rcsb.org/pdb/explore.do?structureId=2VNO">RCBS Protein Data Bank</a> | ||
| + | </p> | ||
| + | </p><img src="https://static.igem.org/mediawiki/2014/1/11/Tue2014_2VNO.jpg"style="width: 400px;"> | ||
| + | <p id="picTextCenter">Figure 4: Endo-β-galactosidase (2VNO) from <i>Clostridium perfringens</i> </p> | ||
</div> | </div> | ||
Latest revision as of 23:57, 17 October 2014
Enzymes
Three different glycosidases were used during our project. All three enzymes target galactose and its derivatives like N-acetylgalactosamine in oligosaccharides and are therefore suitable for modification of the ABO-antigens on the surface of erythrocytes. The described enzymes are capable of cleaving off selectively the characteristic structures of the A and B antigen, thereby generating the H antigen and structures similar to this oligosaccharide, which is characteristic for blood type O.
The α-N-acetylgalactosaminidase (NAGA) from Elizabethkingia meningoseptica is an enzyme which is capable of cleaving off the N-acetylgalactosamine residue of the A antigen. This enzyme consists of 448 amino acids and weights about 50.5 kDa. The structure is determined and can be found in the RCSB Protein Data Base.
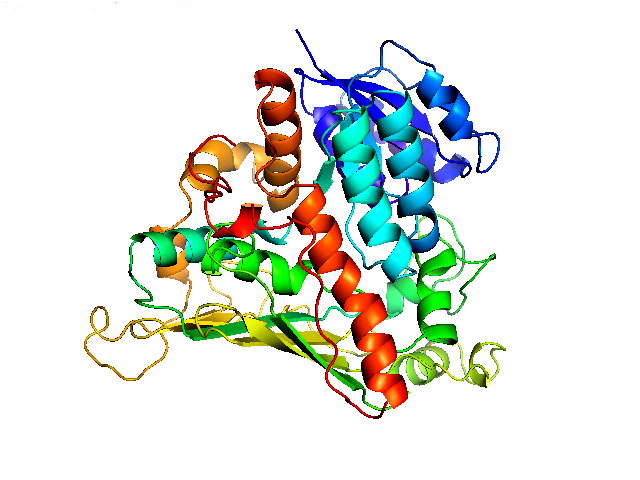
Figure 1: α-N-acetylgalactosaminidase (2IXA) from Elizabethkingia meningoseptica
The NAGA uses an unusual mechanism to achieve its reaction. A catalytic NAD+ accepts a hydride ion from C3 of the N-acetylgalactosamine which leads to formation of an carbonyl group at this position. The carbonyl group at C3 enables the abstraction of a proton at C2 by stabilizing the temporary negative charge. The negative charge leads to the possibility to release the residual carbohydrates as an alcoholate and replacing them with an hydroxide ion. The catalytic cycle is finished by the regeneration of the NAD+ through reduction of the carbonyl function at C3.
Figure 2: Mechanism of the NAGA according to Liu et al.

Figure 3: Oxidation at C3 improves the ability to deprotonate C2
α-Galactosidase (aGal) from Bacteroides fragilis is able to cleave off the terminal galactose residue of the B antigen. The enzyme is 589 amino acids long and weighs 66.12 kDa. The structure of the enzyme is not solved up to today. Kinetic properties were determined in the publication of Liu et al. (2007).
The endo-β-galactosidase (EABase) from Clostridium perfringens is able to accept the A and the B antigen as substrate and attacks the bond between galactose and N-acetylglucosamine. As a consequence, EABase releases the blood group trisaccharides and generates a precursor of the blood antigen H. The enzyme is 804 amino acids long and weights 91.25 kDa. The structure of the binding module of this enzyme is determined and can be accessed in the RCBS Protein Data Bank

Figure 4: Endo-β-galactosidase (2VNO) from Clostridium perfringens
 "
"