Team:ETH Zurich/lab/biobrick/used1
From 2014.igem.org
(→Characterization of the promoter's basal leakiness to LuxR in the absence of 3OC6-HSL) |
|||
| (9 intermediate revisions not shown) | |||
| Line 12: | Line 12: | ||
== Background information == | == Background information == | ||
| - | We used an ''E. coli'' TOP10 strain transformed with two medium copy plasmids (about 15 to 20 copies per plasmid and cell). The first plasmid contained the commonly used p15A origin of replication, a kanamycin resistance gene, and promoter [http://parts.igem.org/Part:BBa_R0062 pLuxR (BBa_R0062)] followed by [http://parts.igem.org/Part:BBa_B0034 RBS (BBa_B0034)] and superfolder green fluorescent protein (sfGFP). In general, for spacer and terminator sequences the parts [http://parts.igem.org/Part:BBa_B0040 BBa_B0040] and [http://parts.igem.org/Part:BBa_B0015 BBa_B0015] were used, respectively. The second plasmid contained the pBR322 origin (pMB1), which yields a stable two-plasmid system together with p15A, an ampicillin resistance gene, one of three promoters chosen from the [http://parts.igem.org/Promoters/Catalog/Anderson Anderson promoter collection] followed by [http://parts.igem.org/Part:BBa_C0062 luxR (BBa_C0062)]. The detailed regulator construct design and full sequences (piG0041, piG0046, piG0047) are [https://2014.igem.org/Team:ETH_Zurich/lab/sequences available here]. | + | We used an ''E. coli'' TOP10 strain transformed with two medium copy plasmids (about 15 to 20 copies per plasmid and cell). The first plasmid contained the commonly used p15A origin of replication, a kanamycin resistance gene, and promoter [http://parts.igem.org/Part:BBa_R0062 pLuxR (BBa_R0062)] followed by [http://parts.igem.org/Part:BBa_B0034 RBS (BBa_B0034)] and superfolder green fluorescent protein (sfGFP). In general, for spacer and terminator sequences the parts [http://parts.igem.org/Part:BBa_B0040 BBa_B0040] and [http://parts.igem.org/Part:BBa_B0015 BBa_B0015] were used, respectively. The second plasmid contained the pBR322 origin (pMB1), which yields a stable two-plasmid system together with p15A, an ampicillin resistance gene, and one of three promoters chosen from the [http://parts.igem.org/Promoters/Catalog/Anderson Anderson promoter collection] followed by [http://parts.igem.org/Part:BBa_C0062 luxR (BBa_C0062)]. The detailed regulator construct design and full sequences (piG0041, piG0046, piG0047) are [https://2014.igem.org/Team:ETH_Zurich/lab/sequences available here]. |
=== Experimental Set-Up === | === Experimental Set-Up === | ||
| - | The above described ''E. coli'' TOP10 strain was grown overnight in Lysogeny Broth (LB) containing kanamycin (50 μg/mL) and ampicillin (200 μg/mL) to an OD<sub>600< | + | The above described ''E. coli'' TOP10 strain was grown overnight in Lysogeny Broth (LB) containing kanamycin (50 μg/mL) and ampicillin (200 μg/mL) to an OD<sub>600</sub> of about 1.5 (37 degrees Celsius, 220 rpm). As a reference, a preculture of the same strain lacking the sfGFP gene was included for each assay. The cultures were then diluted 1:40 in fresh LB containing the appropriate antibiotics and measured in microtiter plate format on 96-well plates (200 μL culture volume) for 10 h at 37 degrees Celsius with a Tecan infinite M200 PRO plate reader (optical density measured at 600 nm; fluorescence with an excitation wavelength of 488 nm and an emission wavelength of 530 nm). |
| - | + | ||
=== Modeling leakiness === | === Modeling leakiness === | ||
| Line 57: | Line 56: | ||
== Results == | == Results == | ||
| + | {|class="wikitable" style="background-color: white; text-align:center; width:auto; margin: auto;" | ||
| + | |+'''Table 1''' Crosstalk matrix for the promoter pLux ([http://parts.igem.org/Part:BBa_R0062:Experience BBa_R0062]) | ||
| + | |colspan="4" style='font-size:10pt';text-align:left| | ||
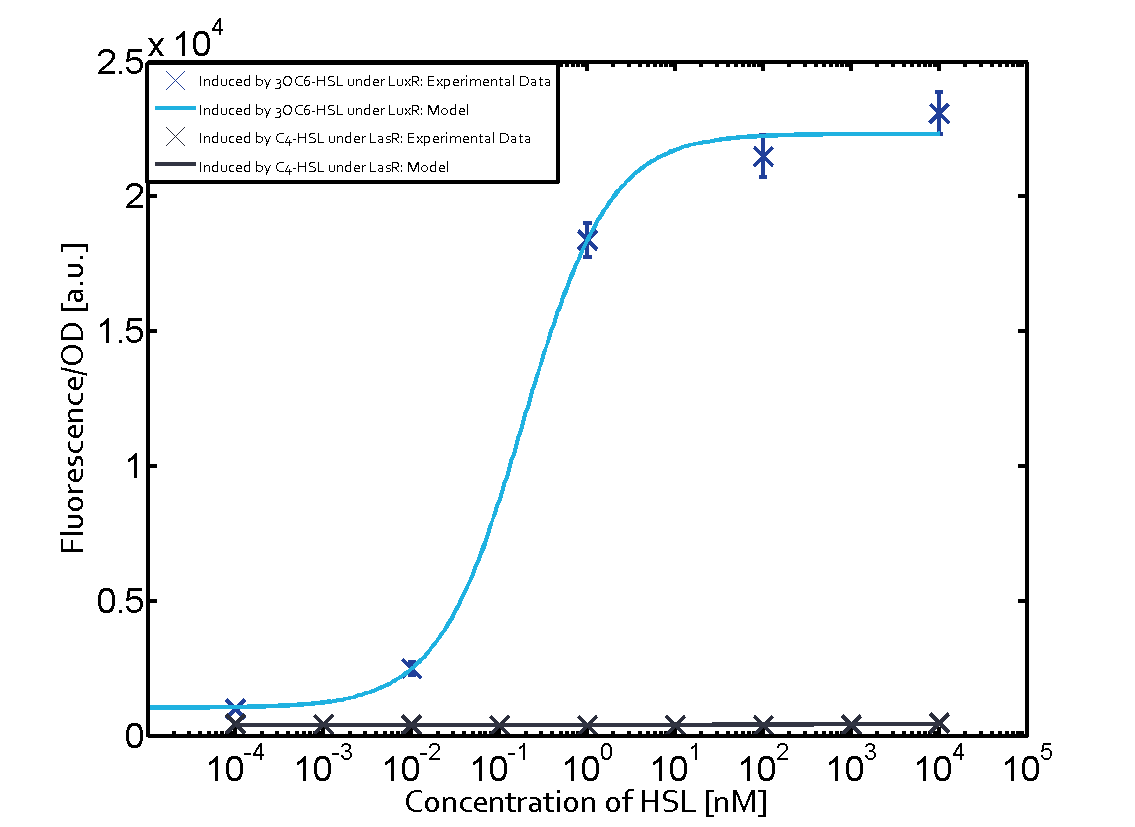
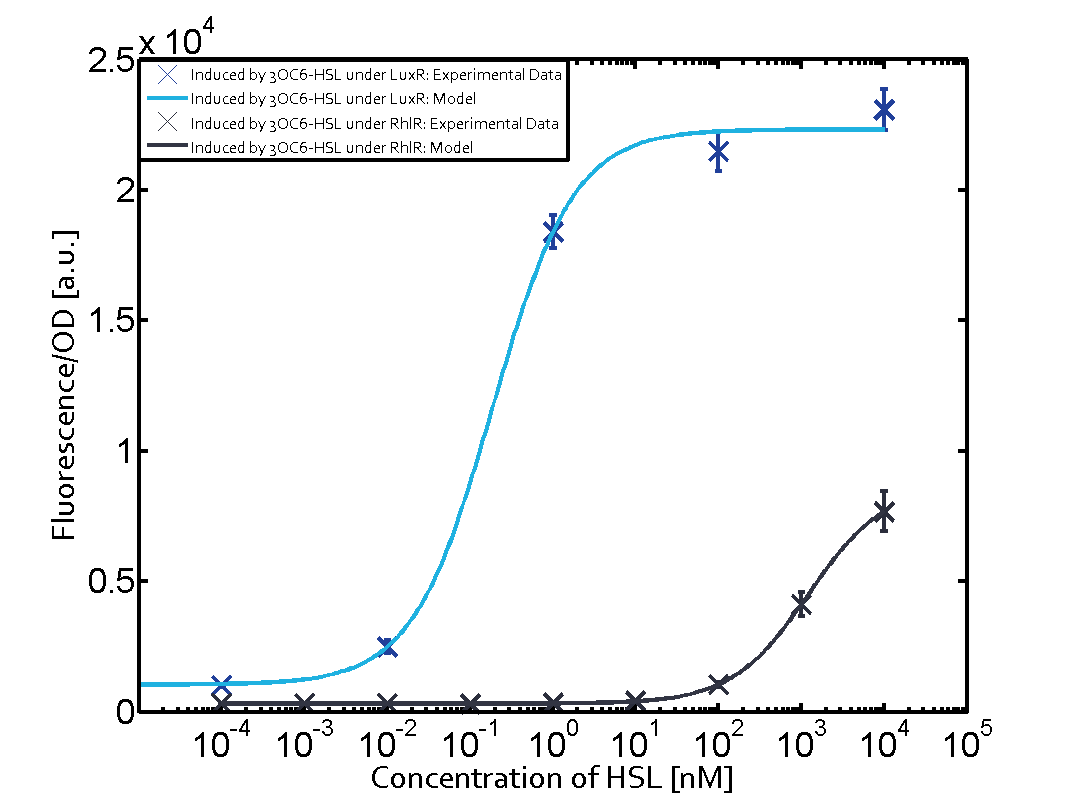
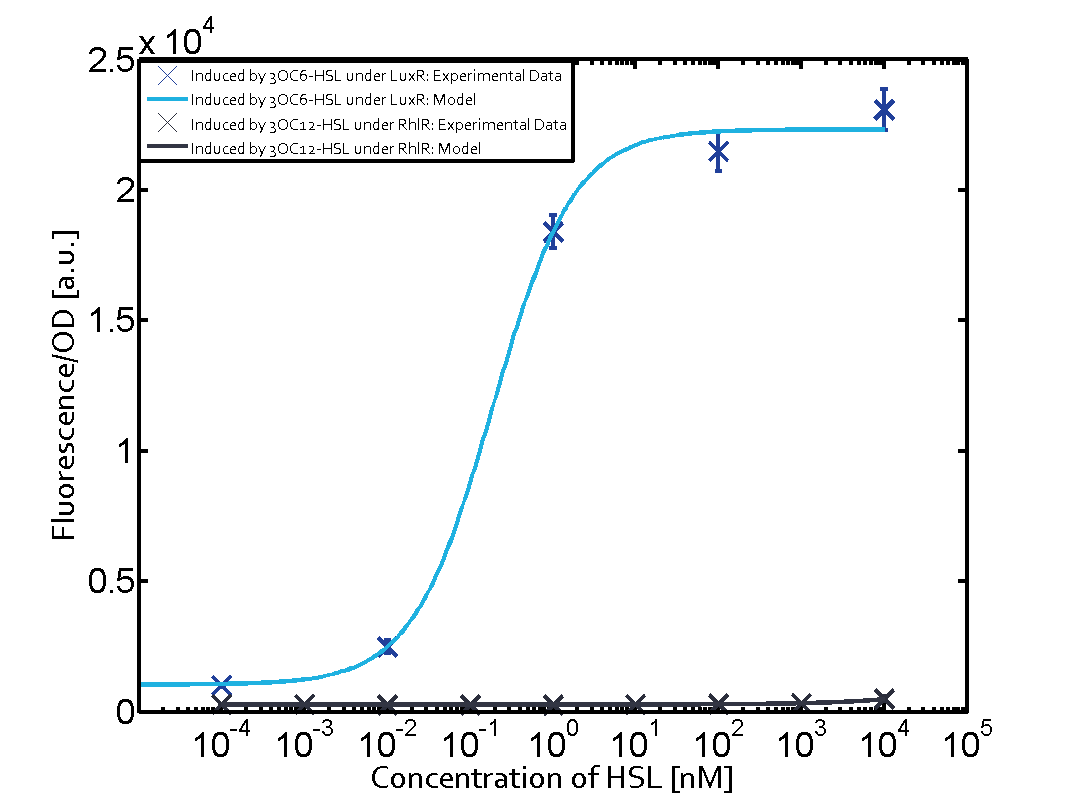
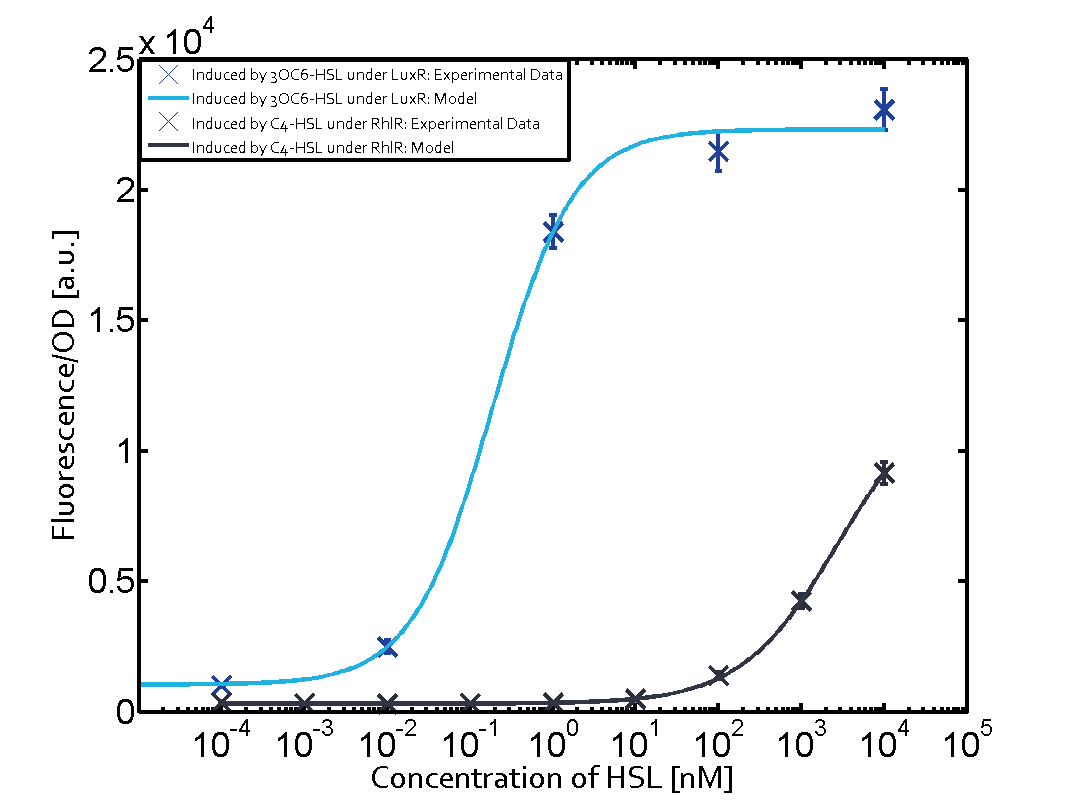
| + | In all the measurements conducted to create this matrix the [http://parts.igem.org/Part:BBa_R0062 promoter pLux] was the basis and was induced in six different variations shown. | ||
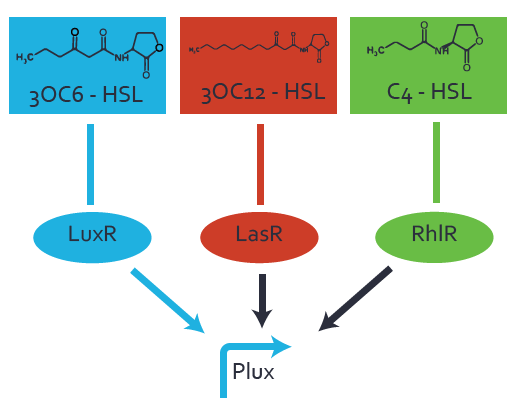
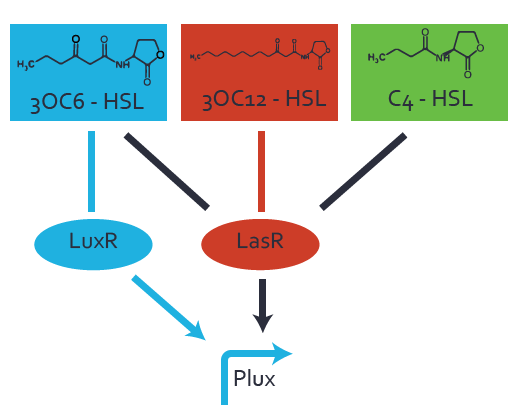
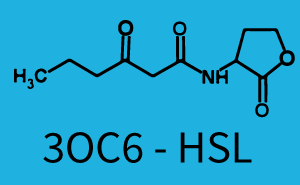
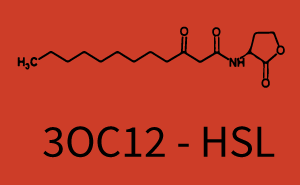
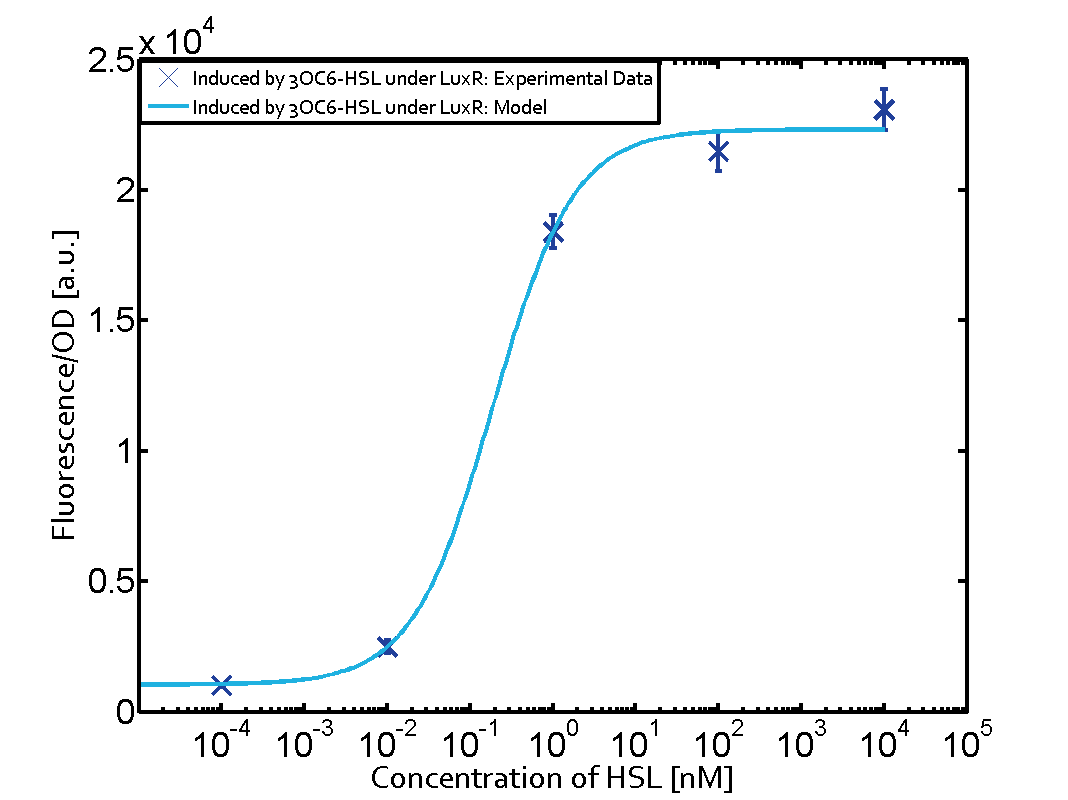
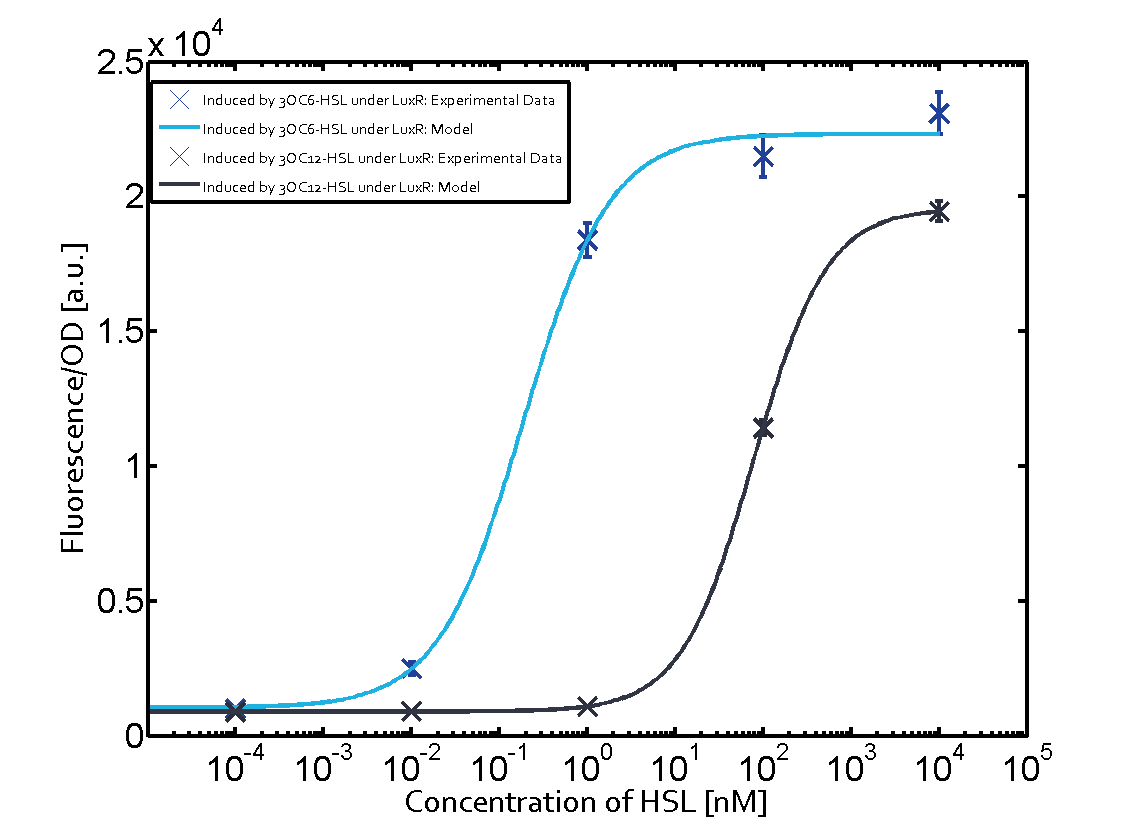
| + | The dark blue points in the graph top left show the activation of gene expression when [http://parts.igem.org/Part:BBa_R0062 pLux] is induced by 3OC6-HSL (Lux-AHL) binding to the corresponding [http://parts.igem.org/Part:BBa_C0062 LuxR regulator]. The observed transition occurs at a concentration of approximately 1 nM of 3OC6-HSL. The light-blue curve plotted shows modeling data of [http://parts.igem.org/Part:BBa_R0062 pLux] induced by 3OC6-HSL (Lux-AHL) binding to the corresponding [http://parts.igem.org/Part:BBa_C0062 LuxR regulator]. This curve from the model and the dark blue data points obtained from experiments were plotted as a reference in all the other graphs describing [http://parts.igem.org/Part:BBa_R0062 pLux]. Crosstalk can be observed for the cases where the 3OC12-HSL (Las-AHL) binds the [http://parts.igem.org/Part:BBa_C0062 LuxR regulator]. | ||
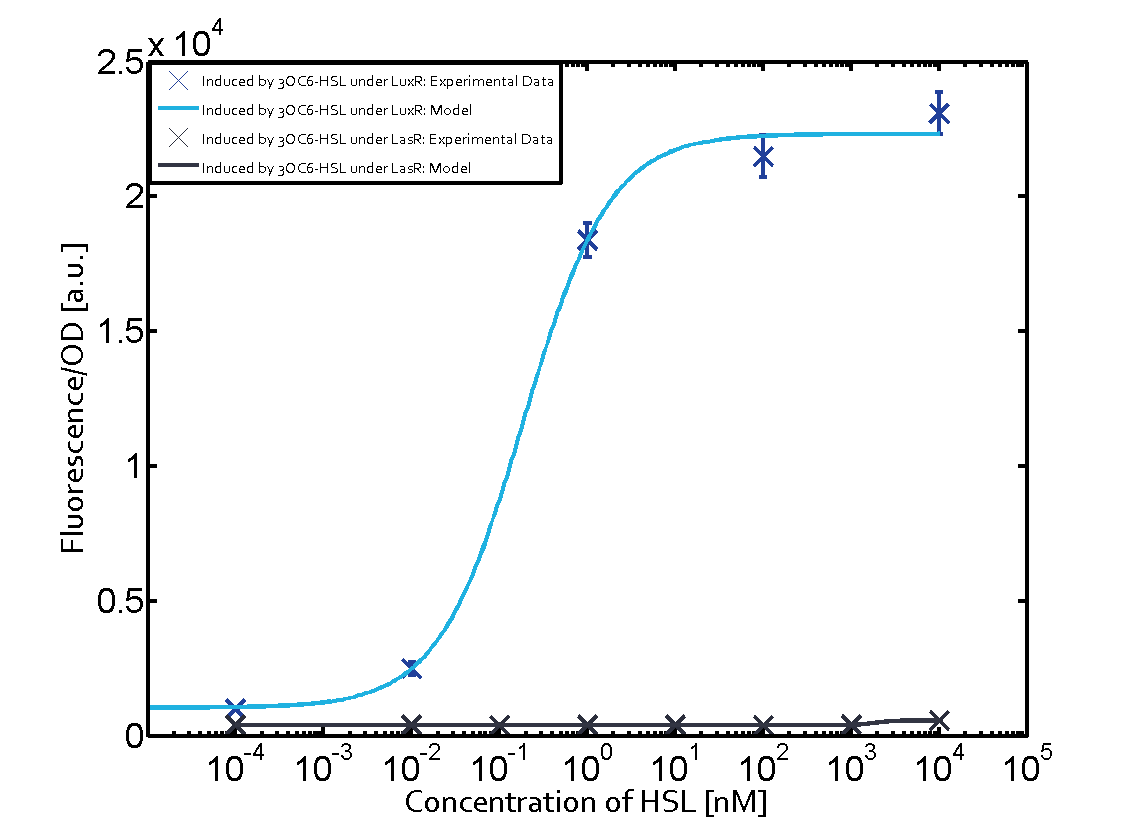
| + | Additionally for 3OC12-HSL binding to its corresponding [http://parts.igem.org/Part:BBa_C0179 regulator LasR] and then binding to the [http://parts.igem.org/Part:BBa_R0062 pLux] as seen in the middle of the top row and center of the matrix. | ||
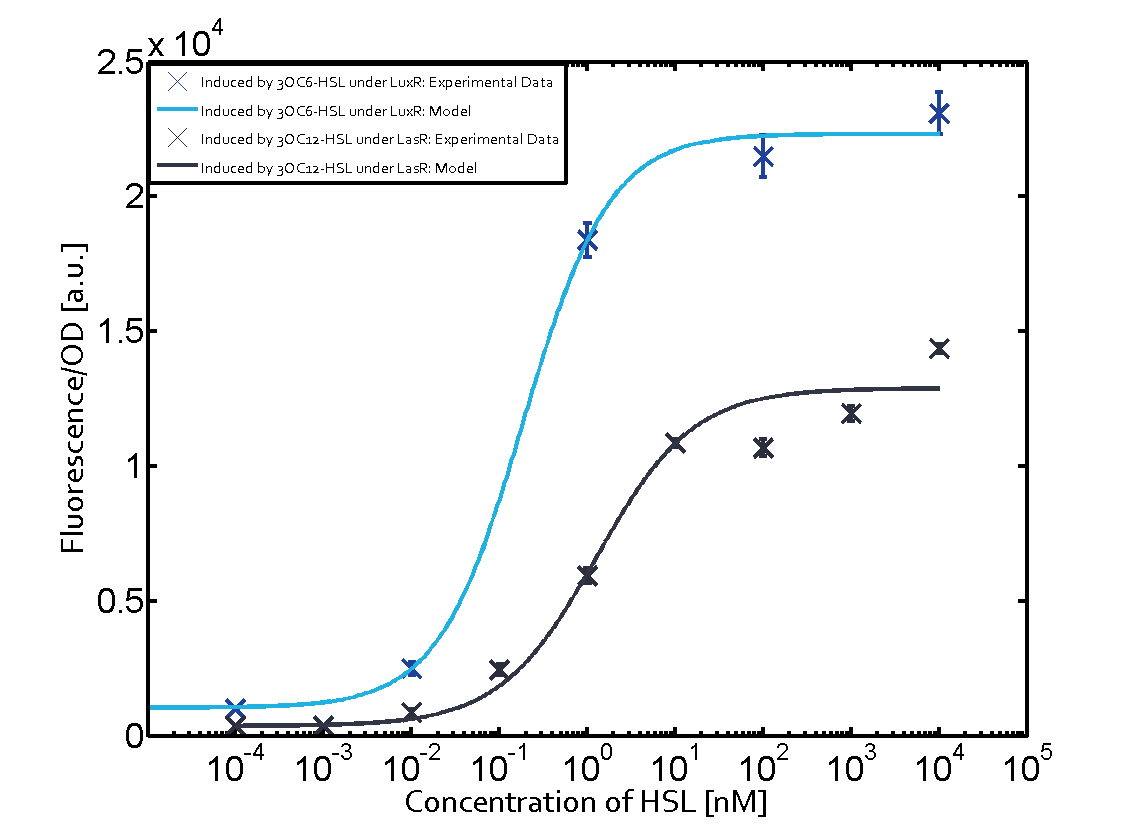
| + | For the case of Las-AHL binding the [http://parts.igem.org/Part:BBa_C0179 regulator LasR] and subsequently the [http://parts.igem.org/Part:BBa_R0062 promoter pLux], the transition occurs at 1 nM and reaches 0.5 fold the fluorescence as [http://parts.igem.org/Part:BBa_R0062 pLux] induced by 3OC6-HSL binding [http://parts.igem.org/Part:BBa_C0062 LuxR]. In the case of 3OC12-HSL binding [http://parts.igem.org/Part:BBa_C0062 LuxR] and inducing the promoter [http://parts.igem.org/Part:BBa_R0062 pLux], the transition is observed at approximately 100 nM and severe crosstalk is observed, meaning that the ON-OFF-ratio is not significantly different from the reference curve. | ||
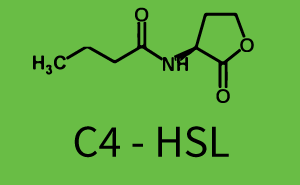
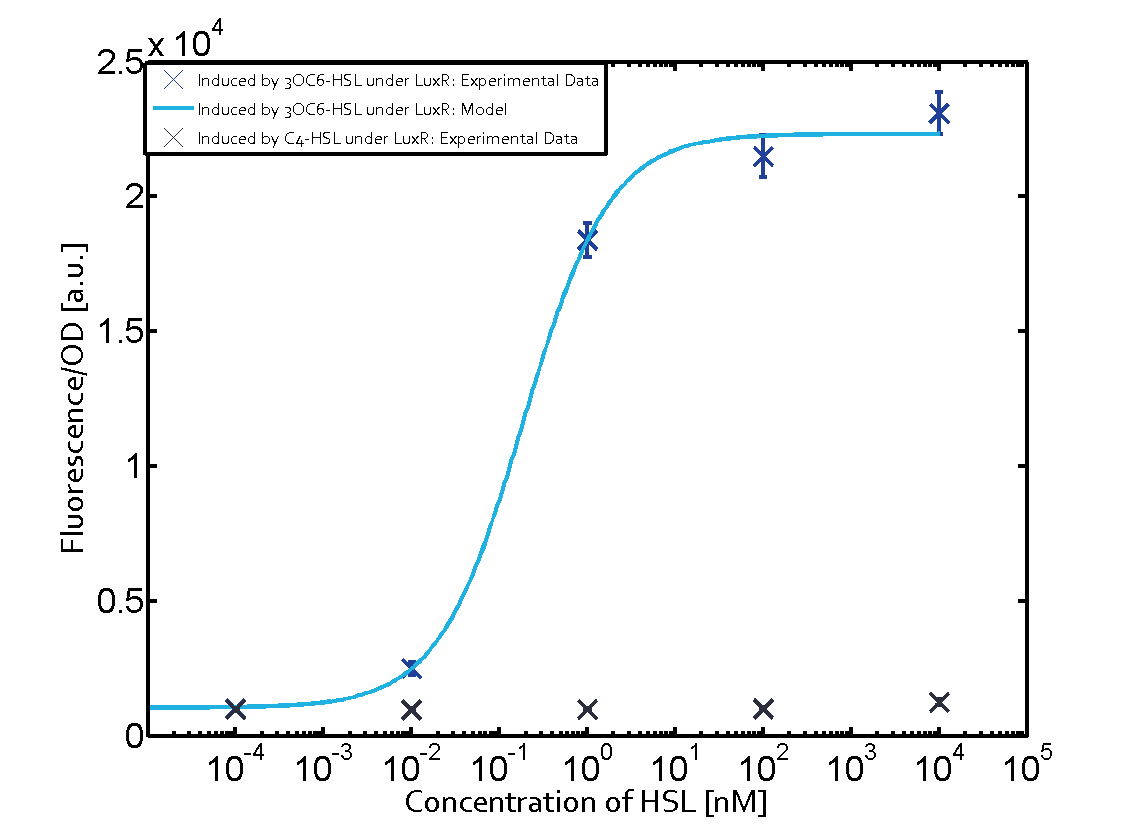
| + | Observation of C4-HSL has shown, that there is no significant crosstalk with the [http://parts.igem.org/Part:BBa_C0062 LuxR regulator] and [http://parts.igem.org/Part:BBa_C0179 LasR regulator] binding C4-HSL and subsequently to [http://parts.igem.org/Part:BBa_R0062 pLux]. This is indicated on top right and middle right graphs. However, [http://parts.igem.org/Part:BBa_C0171 RhlR] induced with its corresponding inducer (C4-HSL) binds to [http://parts.igem.org/Part:BBa_R0062 pLux] and activates expression of GFP at about 100 nM. | ||
| + | |- | ||
| + | |style="width:200px"|[[File:ETH_Zurich_2014_qs-table_CornerLux.png|100px|link=http://parts.igem.org/Part:BBa_C0062:Experience]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_3OC6-HSL.png|100px|link=http://parts.igem.org/Part:BBa_C0062:Experience]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_3OC12-HSL.png|100px|link=http://parts.igem.org/Part:BBa_C0062:Experience]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_C4-HSL.png|100px|link=http://parts.igem.org/Part:BBa_C0062:Experience]] | ||
| + | |- | ||
| + | |[[File:ETH_Zurich_2014_qs-table_LuxR.png|100px|link=http://parts.igem.org/Part:BBa_C0062:Experience]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxRef.png|200px]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxLuxRLasAHL.png|200px]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxLuxRRhlAHL.png|200px]] | ||
| + | |- | ||
| + | |[[File:ETH_Zurich_2014_qs-table_LasR.png|100px|link=http://parts.igem.org/Part:BBa_C0179:Experience]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxLasRLuxAHL.png|200px]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxLasRLasAHL.png|200px]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxLasRRhlAHL.png|200px]] | ||
| + | |- | ||
| + | |[[File:ETH_Zurich_2014_qs-table_RhlR.png|100px|link=http://parts.igem.org/Part:BBa_C0171:Experience]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxRhlRLuxAHL.png|200px]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxRhlRLasAHL.png|200px]] | ||
| + | |[[File:ETH_Zurich_2014_qs-table_PluxRhlRRhlAHL.png|200px]] | ||
| + | |} | ||
|}; | |}; | ||
Latest revision as of 03:18, 18 October 2014
|
<partinfo>BBa_R0062 AddReview 4</partinfo> ETH Zurich 2014 |
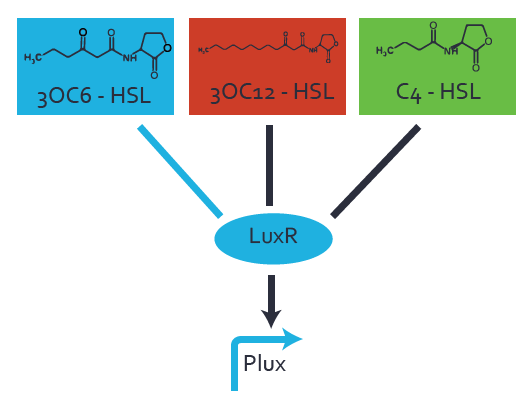
Characterization of the promoter's basal leakiness to LuxR in the absence of 3OC6-HSLThe amount of regulator [http://parts.igem.org/Part:BBa_C0062:Experience LuxR (BBa_C0062)] in the system was shown to influence the pLuxR promoter's basal expression or leakiness. By using the three different constitutive promoters [http://parts.igem.org/Part:BBa_J23100:Experience BBa_J23100], [http://parts.igem.org/Part:BBa_J23109:Experience BBa_J23109], and [http://parts.igem.org/Part:BBa_J23111:Experience BBa_J23111] for the production of LuxR we have measured this effect in terms of fluorescence. Background informationWe used an E. coli TOP10 strain transformed with two medium copy plasmids (about 15 to 20 copies per plasmid and cell). The first plasmid contained the commonly used p15A origin of replication, a kanamycin resistance gene, and promoter [http://parts.igem.org/Part:BBa_R0062 pLuxR (BBa_R0062)] followed by [http://parts.igem.org/Part:BBa_B0034 RBS (BBa_B0034)] and superfolder green fluorescent protein (sfGFP). In general, for spacer and terminator sequences the parts [http://parts.igem.org/Part:BBa_B0040 BBa_B0040] and [http://parts.igem.org/Part:BBa_B0015 BBa_B0015] were used, respectively. The second plasmid contained the pBR322 origin (pMB1), which yields a stable two-plasmid system together with p15A, an ampicillin resistance gene, and one of three promoters chosen from the [http://parts.igem.org/Promoters/Catalog/Anderson Anderson promoter collection] followed by [http://parts.igem.org/Part:BBa_C0062 luxR (BBa_C0062)]. The detailed regulator construct design and full sequences (piG0041, piG0046, piG0047) are available here. Experimental Set-UpThe above described E. coli TOP10 strain was grown overnight in Lysogeny Broth (LB) containing kanamycin (50 μg/mL) and ampicillin (200 μg/mL) to an OD600 of about 1.5 (37 degrees Celsius, 220 rpm). As a reference, a preculture of the same strain lacking the sfGFP gene was included for each assay. The cultures were then diluted 1:40 in fresh LB containing the appropriate antibiotics and measured in microtiter plate format on 96-well plates (200 μL culture volume) for 10 h at 37 degrees Celsius with a Tecan infinite M200 PRO plate reader (optical density measured at 600 nm; fluorescence with an excitation wavelength of 488 nm and an emission wavelength of 530 nm). Modeling leakinessResultsCharacterization of the promoter's sensitivity to 3OC6-HSL depending on LuxR concentrationBackground informationSystems consideredModeling promoter's sensitivityResultsCharacterization of two-level crosstalk on the promoterBackground informationSystem consideredModeling crosstalkFirst-order crosstalkFirst Level crosstalk: LuxR binds to different HSL and activates the promoterSecond Level crosstalk: other regulatory proteins, like LasR, bind to their natural HSL substrate and activates the promoterSecond order crosstalk: Combination of both cross-talk levelsOther regulatory proteins, like LasR, bind to different HSL and activates the promoter Results
|
 "
"