Team:Marburg:Project:Tumor
From 2014.igem.org
| (50 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
CancerSURF | CancerSURF | ||
{{Team:Marburg/Template:StartContinue}} | {{Team:Marburg/Template:StartContinue}} | ||
| + | {{Team:Marburg/Template:Submenu:Project:Cancer}} | ||
| + | <html><a name="intro"><h2>Introduction</h2></a></html> | ||
| - | DARPins ( | + | DARPins (Designed Ankyrin Repeat Proteins) serve as high potential next generation protein drugs. They are derived from natural ankyrin repeat proteins that contain |
| - | protein drugs. They are derived from natural ankyrin repeat proteins that | + | ankyrin repeats, the most common protein-protein interaction motif which are predominantly found in eukaryotic proteins (Al-Khodor et al. 2010). Also, the genomes of |
| - | common protein-protein interaction motif | + | various pathogenic or symbiotic bacteria and eukaryotic viruses contain several genes encoding ankyrin repeat proteins. Typically, two to four library modules, each |
| - | ( | + | corresponding to one ankyrin repeat of 33 amino acids with seven variable positions, are encased by two caps (N- and C-; Figure 1A). Additionally to the resulting high |
| - | viruses contain several genes encoding ankyrin repeat proteins. Typically, two to four | + | diversity, these repetitive modules are extremely small compared to conventional IgG antibodies, thus preventing tissue penetration. They show no effector |
| - | library modules, each corresponding to one ankyrin repeat of 33 amino acids with seven | + | function and can also exert allosteric inhibition mechanisms. |
| - | variable positions, are encased by two caps (N- and C- | + | |
| - | + | ||
| - | conventional IgG antibodies, preventing | + | |
| - | + | ||
<html> | <html> | ||
| Line 20: | Line 18: | ||
<img src="https://static.igem.org/mediawiki/2014/8/8d/Mr_cancersurf_1.png" alt="" /> | <img src="https://static.igem.org/mediawiki/2014/8/8d/Mr_cancersurf_1.png" alt="" /> | ||
<span class="caption"> | <span class="caption"> | ||
| - | Figure 1: (A) Scheme of the DARPin library design. | + | <b>Figure 1: (A)</b> Scheme of the DARPin library design. |
| - | B) Tetrameric StrepDARPidin construct. Four DARPin | + | <b>(B)</b> Tetrameric <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> |
| - | + | construct. Four DARPin Ec1 molecules were fused via linker peptides to a Streptavidin protein. At the N-terminus | |
| - | + | the Ec1 DARPin is fused to a polyhistidine-Tag (His-Tag) and the C-terminus prevents the linkage to the Streptavidin monomer. | |
| - | + | Adapted from Stumpp et al. 2008. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</span> | </span> | ||
</div> | </div> | ||
</html> | </html> | ||
| - | CancerSURF combines the scaffold of a tetrameric Streptavidin | + | CancerSURF combines the scaffold of a tetrameric Streptavidin to increase the specificity of a DARPin to detect a prominent tumor surface marker of many epithelial-derived |
| - | of | + | cancer cells (like lung and colon cancer cells), called EpCAM (epithelial cell adhesion molecule; Went et al. 2006). EpCAM is expressed by every |
| - | adhesion molecule). We planned to fuse an EpCAM-binding DARPin Ec1 with a tetrameric Streptavidin | + | epithelial cell on the basolateral side in a specific ratio while being overexpressed on the surface irregularly by transformed tumor cells (Figure 2). We planned |
| - | increasing the local concentration of the construct for targeting EpCAM-positive tumor cells. In | + | to fuse an EpCAM-binding DARPin Ec1 (Stefan et al. 2011) with a tetrameric Streptavidin increasing the local concentration of the construct for targeting EpCAM-positive |
| - | the next step, we wanted to combine the high density of epitopes offered by the filament of the | + | tumor cells. In the next step, we wanted to combine the high density of epitopes offered by the filament of the bacterial flagellum as a scaffold with the modular |
| - | bacterial flagellum as a scaffold with the modular advantages of a Streptavidin-Strep-Tag-system. | + | advantages of a Streptavidin-Strep-Tag-system. |
| - | + | ||
<html><center> | <html><center> | ||
| - | <div style="margin: 0 auto; width: | + | <div style="margin: 0 auto; width: 75%;"> |
| - | <img src="https://static.igem.org/mediawiki/2014/5/53/MR_epithelial_cells.png" alt="scheme" width=" | + | <img src="https://static.igem.org/mediawiki/2014/5/53/MR_epithelial_cells.png" alt="scheme" width="47%" /> |
| - | <img src="https://static.igem.org/mediawiki/2014/4/42/MR_transformed-cells.png" alt="scheme" width=" | + | <img src="https://static.igem.org/mediawiki/2014/4/42/MR_transformed-cells.png" alt="scheme" width="47%" /> |
| - | + | <span class="caption" style="text-align:justify;width: 94%;"> | |
| - | <span class="caption" style="text-align:justify;width: | + | <b>Figure 2: Transformation of epithelial-derived cancer cells overexpressing EpCAM.</b> |
| - | Figure 2: | + | |
</span> | </span> | ||
</div> | </div> | ||
| Line 53: | Line 45: | ||
</html> | </html> | ||
| - | <html><h2><a name="production">Production of a DARPin-Strep module for specific recognition of EpCAM | + | <html><h2><a name="production">Production of a DARPin-Strep module for specific recognition of EpCAM-positive epithelial-derived cancer cells</a></h2></html> |
| - | To address the goal of creating a | + | To address the goal of creating a CancerSURF for efficient and rapid targeting of cancer cells, we first had to synthesize a Strep-DARPin fusion construct |
| - | cells, we first had to synthesize a Strep-DARPin fusion construct (StrepDARPidin). | + | (<html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html>). |
| - | Template DNA for the tetrameric StrepDARPidin construct was commercially synthesized by IDT | + | Template DNA for the tetrameric <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> construct was commercially |
| - | (Integrated DNA Technologies) and cloned into a pET-plasmide | + | synthesized by IDT (Integrated DNA Technologies) and cloned into a pET-plasmide in order to |
| - | in ''E. | + | overexpress the protein in ''E.coli'' BL21 (DE3). Since we discovered that <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| - | bodies, | + | is not soluble when overexpressed and remains in inclusion bodies, we had to apply |
| - | After refolding the protein was purified in a two-step process: Ni-NTA-affinity purification and | + | a refolding protocol to (re)-gain <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| - | size-exclusion chromatography (Figure 3). The calculated protein mass of the monomeric StrepDARPidin | + | in sufficient amounts (Howarth and Ting, 2008). After refolding, the protein was purified in a two-step process: |
| - | is 31 kDa. The tetramer should have a size of 124 kDa and is visible above the highest marker line | + | Ni-NTA-affinity purification and size-exclusion chromatography (Figure 3). The calculated protein mass of the monomeric |
| - | (116 kDa; Figure 3). | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| + | is 31 kDa. The tetramer should | ||
| + | have a size of 124 kDa and is visible above the highest marker line (116 kDa; Figure 3). | ||
| Line 71: | Line 65: | ||
<img src="https://static.igem.org/mediawiki/2014/2/2b/Mr_cancersurf_2.png" alt="" /> | <img src="https://static.igem.org/mediawiki/2014/2/2b/Mr_cancersurf_2.png" alt="" /> | ||
<span class="caption"> | <span class="caption"> | ||
| - | Figure 3: | + | <b>Figure 3: |
| - | Purification of StrepDARPidin. Size-exclusion chromatogram and | + | Purification of <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>.</b> Size-exclusion chromatogram and |
| - | coomassie stained SDS-PAGE of purified StrepDARPidin. Upper SDS-PAGE: | + | coomassie stained SDS-PAGE of purified <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. Upper SDS-PAGE: |
Fractions taken from Ni-NTA purification. M: marker, L: lysate, FT: flow through, | Fractions taken from Ni-NTA purification. M: marker, L: lysate, FT: flow through, | ||
W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions taken from size-exclusion | W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions taken from size-exclusion | ||
| - | fractions. Samples on the | + | fractions. Samples on the right side have been cooked prior to loading showing the monomeric |
| - | StrepDARPidin. | + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. |
</span> | </span> | ||
</div> | </div> | ||
</html> | </html> | ||
| + | Protein containing fractions were analyzed on a coomassie stained SDS-PAGE and refolding was verified by incubating the eluate for 10 min at 95 °C (Figure 3). The peak | ||
| + | fractions after size-exclusion were pooled and concentrated for further analysis. | ||
| - | + | In the next step, we designed an assay to test the functionality and binding specificity of the engineered | |
| - | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> | |
| - | + | to EpCAM on lung and colon cancer cells. Colon | |
| - | + | cancer cells (Caco-2) and lung cancer cells (A549) which are known to highly express EpCAM (Maaser and Borlak, 2008) were treated with | |
| - | In the next step | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| - | + | in serial dilutions. | |
| - | cells (A549) were treated with StrepDARPidin in | + | After washing, they were incubated with anti-His-antibody-Alexa488 conjugate. Fibroblasts (3T3 wild type cells) were used as EpCAM-negative control. Fluorescence was |
| - | with anti-His-antibody-Alexa488 conjugate. Fibroblasts (3T3 cells) were used as control. Fluorescence | + | quantified at 520 nm (Figure 4C) with a fluorometer. |
| - | + | ||
| - | + | ||
| - | + | ||
<html> | <html> | ||
| Line 99: | Line 92: | ||
<img src="https://static.igem.org/mediawiki/2014/5/54/Mr_cancersurf_3.png" alt="" /> | <img src="https://static.igem.org/mediawiki/2014/5/54/Mr_cancersurf_3.png" alt="" /> | ||
<span class="caption"> | <span class="caption"> | ||
| - | Figure 4: (A) Immunofluorescent staining of Caco-2 and 3T3 cells incubated with | + | <b>Figure 4: (A)</b> Immunofluorescent staining of Caco-2 and 3T3 cells incubated with |
| - | StrepDARPidin protein. Both cell lines were stained with | + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> protein. |
| - | + | Both cell lines were stained with phalloidin (red) conjugated with rhodamine (F-Actin) and Alexa488 (green) conjugated anti-His-antibody | |
| - | + | (<a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>) in presence of 25 µM | |
| - | of A549 cells incubated with StrepDARPidin protein. Cells were stained with | + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. The nucleus was stained with DAPI (blue). Scale bar represents 5 µm. <b>(B)</b> |
| - | + | Immunofluorescent staining of A549 cells incubated with | |
| - | in presence of | + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> protein. Cells were stained with rhodamine (red) conjugated phalloidin |
| - | + | (F-Actin) and Alexa488 (green) conjugated anti-His antibody (<a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>) | |
| - | anti-His-antibody in A549 and Caco-2 (epithelial) cells was normalized to 3T3 (interstitial) | + | in presence of 25 µM <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. The nucleus was stained with DAPI (blue). The control |
| - | + | was stained in | |
| + | absence of <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. Scale bar represents 5 µm. <b>(C)</b> Binding specificity of | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> to epithelial-derived cancer cell lines. Fluorescence of Alexa488-coupled | ||
| + | anti-His-antibody in A549 and Caco-2 (epithelial) cells was normalized to 3T3 (interstitial) cells. <b>(D)</b> Fluorescence intensities of Caco-2 and 3T3 cells in comparison. | ||
</span> | </span> | ||
</div> | </div> | ||
</html> | </html> | ||
| - | The assay confirmed the optimal concentration of StrepDARPidin | + | The assay confirmed the optimal concentration of <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| + | to be 25 µM for the treatment of EpCAM-positive cancer cell lines (Figure 4C). | ||
| - | Next we wanted to investigate if StrepDARPidin can bind to epithelial cells. Therefore we used an immunofluorescence based approach. | + | Next we wanted to investigate if <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| - | Caco-2 and A549 cells were grown on gelatin coated coverslips and incubated with 25 µM StrepDARPidin. As a negative control | + | can bind to epithelial-derived cancer cells. Therefore we used an immunofluorescence based approach. Caco-2 and A549 cells were grown on |
| - | fibroblast cell line 3T3 was used. After fixation with formaldehyde, cells were permeabilized with Triton-X-100. | + | gelatin coated coverslips and incubated with 25 µM <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html>. |
| - | The membrane-associated F-Actin was stained with | + | As a negative control fibroblast cell line 3T3 wild type was used. After fixation with formaldehyde, cells were permeabilized with Triton-X-100. |
| - | green) conjugate was used for the staining of StrepDARPidin. Phalloidin conjugated | + | The membrane-associated F-Actin was stained with phalloidin-rhodamine and the nucleus was stained with DAPI (Figure 4A, B). Anti-His-antibody-Alexa488 (green) |
| - | F-Actin cytoskeleton to follow cell morphology. In Caco-2 and A549 cells a | + | conjugate was used for the staining of <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html>. |
| - | ( | + | Phalloidin conjugated with rhodamine (red) was used for staining of the F-Actin cytoskeleton to follow cell morphology. In Caco-2 and A549 cells a co-localization of |
| - | In Caco-2 cells the total fluorescence intensity ratio membrane/cytoplasm was 2.5x higher compared to 3T3 cells (Figure 4D) | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> with membrane-associated F-Actin could be shown (Figure 4A, B). Some unspecific |
| - | + | His-staining was detected in the nucleus of all cell lines both in presence and absence of | |
| + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html>. | ||
| + | In presence of <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> | ||
| + | both epithelial-derived cancer cell lines showed the highest fluorescence intensity at the cell membrane. In Caco-2 cells the total fluorescence | ||
| + | intensity ratio membrane/cytoplasm was 2.5x higher compared to 3T3 cells (Figure 4D). | ||
| - | We | + | We were able to show that our tetrameric <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| - | + | is capable of binding different EpCAM-positive cancer cell lineages, which already is a remarkable result. Additionally, | |
| - | system for | + | we wanted to increase the local concentration of <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> |
| - | dendritic cells leading to a stimulation of T helper cells. Therefore, employing bacterial flagellar filaments not only drastically | + | molecules and also integrate the activation of the innate immune system for enhancing our CancerSURF. Bacterial surface proteins, such as Flagellin, |
| - | the amount of | + | activate TLR5 (toll-like receptor 5) on dendritic cells, leading to a stimulation of T helper cells. Therefore, employing bacterial flagellar filaments not only drastically |
| + | increases the amount of <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> present on the cancer cell surface but also leads | ||
| + | to a stimulation of the inflammatory immune response (Leigh et al. 2014) which | ||
| + | creates an anti-tumor environment (Tarahomjoo et al. 2013) . | ||
| + | <html> | ||
| + | <div class="figure" style="width:10%"> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/0/02/MR_DC_TLR5.png" alt="" /> | ||
| + | <span class="caption"> | ||
| + | <b>Figure 5: Cellular response after TLR-5 activation by Flagellin.</b> | ||
| + | </span> | ||
| + | </div> | ||
| + | </html> | ||
| - | |||
| - | As described previously enhancing the local concentration of DARPin | + | <html><h2><a name="coupling">Coupling StrepDARPidin to engineered Hag-D2-Strep</a></h2></html> |
| - | Therefore, | + | |
| - | information) with the | + | As described previously, enhancing the local concentration of DARPin molecules is crucial to allow a rapid and efficient targeting of cancer cells. Therefore, |
| - | well as overexpression in | + | B. subtilis Flagellin was engineered carrying an insertion in the D1-domain replacing the small native loop (see SilverSURF for detailed information) with the <i>S. |
| - | + | typhimurium</i> D2-domain and a Strep-Tag. The Hag-D2-Strep <html><a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">(BBa_K1329003)</a></html> was used for | |
| - | (Figure | + | the generation of modified <i>B. subtilis</i> strains as well as overexpression in <i>E. coli</i> BL21 |
| + | (DE3) cells (Figure 6A). As Flagellin tends to form polymers when overexpressed (a natural feature necessary to build up the filament), it was co-purified with its | ||
| + | cognate chaperone FliS, forming a stable and stoichiometric complex on a size-exclusion column (Figure 6A). The fractions of the main peak were pooled and concentrated | ||
| + | to a final volume of 500 µL for further analysis. | ||
| + | |||
<html> | <html> | ||
| Line 145: | Line 160: | ||
<img src="https://static.igem.org/mediawiki/2014/0/07/Mr_cancersurf_4.png" alt="" /> | <img src="https://static.igem.org/mediawiki/2014/0/07/Mr_cancersurf_4.png" alt="" /> | ||
<span class="caption"> | <span class="caption"> | ||
| - | Figure | + | <b>Figure 6: Purification of <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep</a> and binding |
| - | chromatogram of | + | assays with <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. (A)</b> Size-exclusion |
| + | chromatogram of <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep</a> | ||
| + | in complex with its chaperone FliS. Upper SDS-PAGE: Ni-NTA purification. | ||
M: marker, L: lysate, FT: flow through, W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions | M: marker, L: lysate, FT: flow through, W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions | ||
| - | taken from size-exclusion fractions. (B)Streptavidin-and GST-interaction assay using purified | + | taken from size-exclusion fractions. <b>(B)</b> Streptavidin- and GST-interaction assay using purified |
| - | and | + | <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a> |
| - | Strep-beads with | + | and Hag-Strep/FliS. (1) Strep-beads without protein (contaminant protein at 30 kDa). (2) Strep-beads with Hag-Strep/FliS immobilized. (3) |
| - | (C) Interaction of StrepDARPidin and | + | Strep-beads with <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a>. |
| - | (1) | + | (4) GST-beads with Hag-Strep/FliS. (5) GST-beads with <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a>. |
| - | StrepDARPidin. (5) StrepDARPidin. Insoluble fractions: (6) | + | <b>(C)</b> Interaction of <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> and |
| - | + | <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a> | |
| - | and StrepDARPidin (1:1). | + | visualized by competition assays. Soluble fractions: |
| + | (1) Hag-Strep/FliS. (2) <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a>. | ||
| + | (3) Hag-Strep/FliS and <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. (4) | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a> and | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. (5) | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a>. Insoluble fractions: (6) Hag-Strep/FliS and | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> (1:5 dilution). (7) | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a> and | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> (1:5 dilution).(8) Hag-Strep/FliS and | ||
| + | <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> (1:1 dilution). | ||
| + | (9) <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep/FliS</a> | ||
| + | and <a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a> (1:1 dilution). | ||
</span> | </span> | ||
</div> | </div> | ||
</html> | </html> | ||
| - | To investigate whether the purified | + | To investigate whether the purified <html><a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep</a></html> |
| - | intact filaments from | + | is able to interact with Streptavidin ''in vitro'' prior to |
| - | immobilized purified | + | labeling intact filaments from <i>B. subtilis</i> flagella, we used Streptavidin-pulldown assays to confirm their functionality. For this we immobilized purified |
| - | variants of Flagellin | + | <html><a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep</a></html> and Hag-Strep on Streptavidin- and GST-beads (Figure 6B). Both engineered |
| - | (Figure 5B, lanes 2 and 3). | + | variants of Flagellin were able to bind to Streptavidin-beads but not GST and thus confirmed that the Strep-Tag is accessible and functional (Figure 5B, lanes 2 and 3). |
| - | + | Furthermore, we wanted to know if our functional | |
| - | This was the last and crucial step that had to be verified before docking of StrepDARPidin to the flagellar filaments | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> is capable of binding to the engineered Flagellin variants. |
| - | could be performed. Purified proteins were mixed in different ratios and precipitation was observed. Samples from the | + | This was the last and crucial step that had to be verified before the docking of |
| - | supernatant (soluble fraction) and the pellet (insoluble fraction) were loaded on a SDS-PAGE showing that the pellet | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> to the flagellar filaments could be performed. |
| - | contained a huge amount of StrepDARPidin and the Flagellin variants (Figure 5C). We | + | Purified proteins were mixed in different ratios and precipitation was observed. Samples from the supernatant (soluble fraction) and the |
| - | of Streptavidin with the Strep-Tag leads to an aggregation of | + | pellet (insoluble fraction) were loaded on a SDS-PAGE showing that the pellet contained a huge amount of |
| - | (Figure 5C, lanes 6-9). | + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> and the Flagellin variants (Figure 5C). |
| + | We assumed that the strong interaction of Streptavidin with the Strep-Tag leads to an aggregation of Hag-Strep variants followed by | ||
| + | precipitation (Figure 5C, lanes 6-9). | ||
| + | |||
| + | The D2-Strep variant of Flagellin was also used for replacing the native Flagellin in <i>B. subtilis</i> as described before (see | ||
| + | <html><a href="https://2014.igem.org/Team:Marburg:Project:Silver" target="_blank">SilverSURF</a></html> for detailed information) in order to generate a | ||
| + | strain exposing the Strep-Tag on the surface of the flagellar filament. ''In vitro'' assays confirmed that the | ||
| + | <html><a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep</a></html> was stable and functional but we had no information about the incorporation | ||
| + | of the engineered Flagellin variant into the flagellar filament ''in vivo''. Therefore, we inoculated swimming plates (containing 0.3 % agar) and swarming plates | ||
| + | (containing 0.7 % agar) with <i>B. subtilis</i> wild type and D2-Strep cells. The mutant strain showed only slightly slower spreading on both plates, indicating that both | ||
| + | single cellular and multi cellular movement is not strongly affected (Figure 6E, F). Additionally, we used negative staining transmission electron microscopy and were able | ||
| + | to show that D2-Strep cells are as much flagellated as the wild type (Figure 6A, B). Finally, we can state that our engineered Flagellin variant D2-Strep is fully functional | ||
| + | and incorporated into intact flagellar filaments. | ||
| + | The last step, immobilizing <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> | ||
| + | on purified filaments, is currently finalized in the laboratory. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<html> | <html> | ||
| Line 196: | Line 222: | ||
<img src="https://static.igem.org/mediawiki/2014/e/e0/Mr_cancersurf_5.png" alt="" /> | <img src="https://static.igem.org/mediawiki/2014/e/e0/Mr_cancersurf_5.png" alt="" /> | ||
<span class="caption"> | <span class="caption"> | ||
| - | Figure | + | <b>Figure 7: Electron micrographs of uranyl-acetate stained <i>B. subtilis</i> cells. (A)</b> Whole cell of wild type strain |
| - | 3610 (B) Whole cell of | + | 3610 <b>(B)</b> Whole cell of <a href="http://parts.igem.org/Part:BBa_K1329003" target="_blank">Hag-D2-Strep</a> strain. Scale bar represents 1 μm. |
| - | + | <b>(C)</b> Magnification of flagellar filaments from 3610 wild type strain. Scale bar represents 100 nm <b>(D)</b> Magnification of flagellar filaments | |
| - | from | + | from Hag-D2-Strepstrain. Scale bar represents 300 nm. <b>(E)</b> Swarming assay of both <i>B. subtilis</i> strains. |
| - | (F)Swimming assay of both | + | <b>(F)</b> Swimming assay of both <i>B. subtilis</i> strains. |
</span> | </span> | ||
</div> | </div> | ||
</html> | </html> | ||
| + | |||
| + | <html><h2><a name="perspectives">Future perspectives in therapy and diagnostics </a></h2></html> | ||
| + | |||
| + | The CancerSURF is a novel, modular and efficient method to target different cancer cell lineages and opens the gate for new applications in cancer treatment. | ||
| + | The flagellar filament serves as a huge scaffold that can be loaded with different agents of ones own choice. | ||
| + | By fusing the tetrameric Streptavidin to small molecules such as DARPins, a wide range of different applications is imaginable. Combining e.g. | ||
| + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> | ||
| + | and fluorescent proteins fused to Streptavidin, cancer cells could be visualized during clinical surgeries. | ||
| + | Cytotoxic agents in combination with | ||
| + | <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> could directly lead to cancer | ||
| + | cell lysis without causing collateral damage to the surrounding tissues making <html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html> | ||
| + | an imaginable antibody alternative for both future diagnostic and therapeutic perspectives. | ||
| + | |||
| + | <html> | ||
| + | <div class="figure" style="width:90%;"> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/6/6f/MR_tumor_Terminator.png" alt="" /> | ||
| + | <span class="caption"> | ||
| + | <b>Figure 8: Model for possible future applications in lung cancer treatment.</b> | ||
| + | </span> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
| + | <html><a name="improve"><h2>Improve a brick</h2></a></html> | ||
| + | |||
| + | One of the strongest interactions established by biological molecules is the interaction | ||
| + | between Streptavidin and biotin, a cofactor of carboxylases. The property of Streptavidin to | ||
| + | tightly bind substrates in combination with engineered Strep-Tags (peptide-tag) has been | ||
| + | utilized over the years for several approaches, e.g. biochemical and immunohistochemical | ||
| + | methods for protein purification and immunofluorescent stainings. | ||
| + | |||
| + | In 2006, the iGEM team from Harvard used Streptavidin to stain the outer membrane of ''E. coli'' cells. By placing | ||
| + | the <html><a href="http://parts.igem.org/Part:BBa_J36848" target="_blank">Streptavidin</a></html> | ||
| + | coding sequence downstream of a membrane targeting signal sequence and outer membrane protein | ||
| + | under control of an IPTG inducible promoter, the heterologous production in ''E. coli'' was achieved | ||
| + | to selectively stain the outer membrane by biotinylated fluorophores. | ||
| + | |||
| + | Our aim was to use the extremely tight interaction of Streptavidin and the engineered Strep-TagII, | ||
| + | which binds to Streptavidin in the low pico molar range, in combination with | ||
| + | DARPin to selectively target epithelial-derived cancer cells and amplify the interaction by | ||
| + | our Strep-tagged flagellar filament scaffold. Therefore, we designed a codon-optimized | ||
| + | Streptavidin/DARPin chimeric gene (<html><a href="http://parts.igem.org/Part:BBa_K1329000" target="_blank">StrepDARPidin</a></html>) | ||
| + | for the heterologous production in ''E. coli''. This chimeric gene enables the efficient | ||
| + | production of StrepDARPidin in ''E. coli'' and may also serve as a template for the codon optimized Streptavidin gene for future iGEM teams, | ||
| + | thereby improving the existing | ||
| + | Streptavidin biobrick gene in the registry. Additionally, we established a refolding protocol | ||
| + | for the purification of StrepDARPidin and verified its functionality and selectivity by an | ||
| + | immunofluorescent analysis of targeted cancer cells and Flagellin-strep binding assays, | ||
| + | thereby improving the sole Streptavidin by adding the EpCAM binding property to the protein. | ||
| + | |||
| + | <html> | ||
| + | <br> | ||
| + | <hr></html> | ||
| + | |||
| + | |||
| + | Al-Khodor S, Price CT, Kalia A, Abu Kwaik Y (2010). Ankyrin-repeat containing proteins of microbes: a conserved structure with functional diversity.Trends Microbiol. 18, 132–139 10.1016 | ||
| + | |||
| + | Howarth M, TingAY (2008). Imaging proteins in live mammalian cells with biotin ligase and monovalent Streptavidin. Nature Protocols 3, 534 - 545 | ||
| + | |||
| + | Leigh ND, Guanglin Bian, Xilai Ding, Hong Liu, Semra Aygun-Sunar, Lyudmila G. Burdelya, Andrei V. Gudkov, Xuefang Cao (2014). A Flagellin-Derived Toll-Like Receptor 5 Agonist Stimulates Cytotoxic Lymphocyte-Mediated Tumor Immunity. PLoS ONE 9(1): e85587 | ||
| + | |||
| + | Maaser K, Borlak J (2008). A genome-wide expression analysis identifies a network of EpCAM-induced cell cycle regulators.British Journal of Cancer 99,1635 – 1643 | ||
| + | |||
| + | Stefan N, Martin-Killias P, Wyss-Stoeckle (2011). DARPins Recognizing the Tumor-Associated Antigen EpCAM Selected by Phage and Ribosome Display and Engineered for Multivalency. | ||
| + | J. Mol. Biol. (2011) 413, 826–843 | ||
| + | |||
| + | Stumpp MT, Binz HK, Amstutz P. (2008). DARPins a new generation of protein therapeutics.Drug Discov. Today. Aug. 13(15-16):695-701. | ||
| + | |||
| + | Tarahomjoo S (2013). Utilizing bacterial flagellins against infectious diseases and cancers. | ||
| + | Antonie van Leeuwenhoek 105:275–288 | ||
| + | |||
| + | Went P, Vasei M, Bubendorf L (2006). Frequent high-level expression of the Immunotherapeutic targetEp-CAM in colon, stomach, prostate and lung cancers. British Journal of Cancer 94, 128 – 135 | ||
| + | |||
<html> | <html> | ||
| + | <!-- | ||
<div id="animation_cancer"><img src="https://static.igem.org/mediawiki/2014/2/27/Mr_animation_cancerSURF.gif" alt="animation" /></div> | <div id="animation_cancer"><img src="https://static.igem.org/mediawiki/2014/2/27/Mr_animation_cancerSURF.gif" alt="animation" /></div> | ||
| Line 219: | Line 318: | ||
<p style="text-align: center;"><img src="https://static.igem.org/mediawiki/2014/3/3b/MR_strepdarpin_structure.png" alt="scheme" width="40%" style="margin: 0 auto;"></p> | <p style="text-align: center;"><img src="https://static.igem.org/mediawiki/2014/3/3b/MR_strepdarpin_structure.png" alt="scheme" width="40%" style="margin: 0 auto;"></p> | ||
| - | + | --> | |
</html> | </html> | ||
{{Team:Marburg/Template:End}} | {{Team:Marburg/Template:End}} | ||
Latest revision as of 03:33, 18 October 2014
CancerSURF
Introduction
DARPins (Designed Ankyrin Repeat Proteins) serve as high potential next generation protein drugs. They are derived from natural ankyrin repeat proteins that contain ankyrin repeats, the most common protein-protein interaction motif which are predominantly found in eukaryotic proteins (Al-Khodor et al. 2010). Also, the genomes of various pathogenic or symbiotic bacteria and eukaryotic viruses contain several genes encoding ankyrin repeat proteins. Typically, two to four library modules, each corresponding to one ankyrin repeat of 33 amino acids with seven variable positions, are encased by two caps (N- and C-; Figure 1A). Additionally to the resulting high diversity, these repetitive modules are extremely small compared to conventional IgG antibodies, thus preventing tissue penetration. They show no effector function and can also exert allosteric inhibition mechanisms.
 Figure 1: (A) Scheme of the DARPin library design.
(B) Tetrameric StrepDARPidin
construct. Four DARPin Ec1 molecules were fused via linker peptides to a Streptavidin protein. At the N-terminus
the Ec1 DARPin is fused to a polyhistidine-Tag (His-Tag) and the C-terminus prevents the linkage to the Streptavidin monomer.
Adapted from Stumpp et al. 2008.
Figure 1: (A) Scheme of the DARPin library design.
(B) Tetrameric StrepDARPidin
construct. Four DARPin Ec1 molecules were fused via linker peptides to a Streptavidin protein. At the N-terminus
the Ec1 DARPin is fused to a polyhistidine-Tag (His-Tag) and the C-terminus prevents the linkage to the Streptavidin monomer.
Adapted from Stumpp et al. 2008.
CancerSURF combines the scaffold of a tetrameric Streptavidin to increase the specificity of a DARPin to detect a prominent tumor surface marker of many epithelial-derived cancer cells (like lung and colon cancer cells), called EpCAM (epithelial cell adhesion molecule; Went et al. 2006). EpCAM is expressed by every epithelial cell on the basolateral side in a specific ratio while being overexpressed on the surface irregularly by transformed tumor cells (Figure 2). We planned to fuse an EpCAM-binding DARPin Ec1 (Stefan et al. 2011) with a tetrameric Streptavidin increasing the local concentration of the construct for targeting EpCAM-positive tumor cells. In the next step, we wanted to combine the high density of epitopes offered by the filament of the bacterial flagellum as a scaffold with the modular advantages of a Streptavidin-Strep-Tag-system.

 Figure 2: Transformation of epithelial-derived cancer cells overexpressing EpCAM.
Figure 2: Transformation of epithelial-derived cancer cells overexpressing EpCAM.
Production of a DARPin-Strep module for specific recognition of EpCAM-positive epithelial-derived cancer cells
To address the goal of creating a CancerSURF for efficient and rapid targeting of cancer cells, we first had to synthesize a Strep-DARPin fusion construct (StrepDARPidin). Template DNA for the tetrameric StrepDARPidin construct was commercially synthesized by IDT (Integrated DNA Technologies) and cloned into a pET-plasmide in order to overexpress the protein in E.coli BL21 (DE3). Since we discovered that StrepDARPidin is not soluble when overexpressed and remains in inclusion bodies, we had to apply a refolding protocol to (re)-gain StrepDARPidin in sufficient amounts (Howarth and Ting, 2008). After refolding, the protein was purified in a two-step process: Ni-NTA-affinity purification and size-exclusion chromatography (Figure 3). The calculated protein mass of the monomeric StrepDARPidin is 31 kDa. The tetramer should have a size of 124 kDa and is visible above the highest marker line (116 kDa; Figure 3).
 Figure 3:
Purification of StrepDARPidin. Size-exclusion chromatogram and
coomassie stained SDS-PAGE of purified StrepDARPidin. Upper SDS-PAGE:
Fractions taken from Ni-NTA purification. M: marker, L: lysate, FT: flow through,
W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions taken from size-exclusion
fractions. Samples on the right side have been cooked prior to loading showing the monomeric
StrepDARPidin.
Figure 3:
Purification of StrepDARPidin. Size-exclusion chromatogram and
coomassie stained SDS-PAGE of purified StrepDARPidin. Upper SDS-PAGE:
Fractions taken from Ni-NTA purification. M: marker, L: lysate, FT: flow through,
W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions taken from size-exclusion
fractions. Samples on the right side have been cooked prior to loading showing the monomeric
StrepDARPidin.
Protein containing fractions were analyzed on a coomassie stained SDS-PAGE and refolding was verified by incubating the eluate for 10 min at 95 °C (Figure 3). The peak fractions after size-exclusion were pooled and concentrated for further analysis.
In the next step, we designed an assay to test the functionality and binding specificity of the engineered StrepDARPidin to EpCAM on lung and colon cancer cells. Colon cancer cells (Caco-2) and lung cancer cells (A549) which are known to highly express EpCAM (Maaser and Borlak, 2008) were treated with StrepDARPidin in serial dilutions. After washing, they were incubated with anti-His-antibody-Alexa488 conjugate. Fibroblasts (3T3 wild type cells) were used as EpCAM-negative control. Fluorescence was quantified at 520 nm (Figure 4C) with a fluorometer.
 Figure 4: (A) Immunofluorescent staining of Caco-2 and 3T3 cells incubated with
StrepDARPidin protein.
Both cell lines were stained with phalloidin (red) conjugated with rhodamine (F-Actin) and Alexa488 (green) conjugated anti-His-antibody
(StrepDARPidin) in presence of 25 µM
StrepDARPidin. The nucleus was stained with DAPI (blue). Scale bar represents 5 µm. (B)
Immunofluorescent staining of A549 cells incubated with
StrepDARPidin protein. Cells were stained with rhodamine (red) conjugated phalloidin
(F-Actin) and Alexa488 (green) conjugated anti-His antibody (StrepDARPidin)
in presence of 25 µM StrepDARPidin. The nucleus was stained with DAPI (blue). The control
was stained in
absence of StrepDARPidin. Scale bar represents 5 µm. (C) Binding specificity of
StrepDARPidin to epithelial-derived cancer cell lines. Fluorescence of Alexa488-coupled
anti-His-antibody in A549 and Caco-2 (epithelial) cells was normalized to 3T3 (interstitial) cells. (D) Fluorescence intensities of Caco-2 and 3T3 cells in comparison.
Figure 4: (A) Immunofluorescent staining of Caco-2 and 3T3 cells incubated with
StrepDARPidin protein.
Both cell lines were stained with phalloidin (red) conjugated with rhodamine (F-Actin) and Alexa488 (green) conjugated anti-His-antibody
(StrepDARPidin) in presence of 25 µM
StrepDARPidin. The nucleus was stained with DAPI (blue). Scale bar represents 5 µm. (B)
Immunofluorescent staining of A549 cells incubated with
StrepDARPidin protein. Cells were stained with rhodamine (red) conjugated phalloidin
(F-Actin) and Alexa488 (green) conjugated anti-His antibody (StrepDARPidin)
in presence of 25 µM StrepDARPidin. The nucleus was stained with DAPI (blue). The control
was stained in
absence of StrepDARPidin. Scale bar represents 5 µm. (C) Binding specificity of
StrepDARPidin to epithelial-derived cancer cell lines. Fluorescence of Alexa488-coupled
anti-His-antibody in A549 and Caco-2 (epithelial) cells was normalized to 3T3 (interstitial) cells. (D) Fluorescence intensities of Caco-2 and 3T3 cells in comparison.
The assay confirmed the optimal concentration of StrepDARPidin to be 25 µM for the treatment of EpCAM-positive cancer cell lines (Figure 4C).
Next we wanted to investigate if StrepDARPidin can bind to epithelial-derived cancer cells. Therefore we used an immunofluorescence based approach. Caco-2 and A549 cells were grown on gelatin coated coverslips and incubated with 25 µM StrepDARPidin. As a negative control fibroblast cell line 3T3 wild type was used. After fixation with formaldehyde, cells were permeabilized with Triton-X-100. The membrane-associated F-Actin was stained with phalloidin-rhodamine and the nucleus was stained with DAPI (Figure 4A, B). Anti-His-antibody-Alexa488 (green) conjugate was used for the staining of StrepDARPidin. Phalloidin conjugated with rhodamine (red) was used for staining of the F-Actin cytoskeleton to follow cell morphology. In Caco-2 and A549 cells a co-localization of StrepDARPidin with membrane-associated F-Actin could be shown (Figure 4A, B). Some unspecific His-staining was detected in the nucleus of all cell lines both in presence and absence of StrepDARPidin. In presence of StrepDARPidin both epithelial-derived cancer cell lines showed the highest fluorescence intensity at the cell membrane. In Caco-2 cells the total fluorescence intensity ratio membrane/cytoplasm was 2.5x higher compared to 3T3 cells (Figure 4D).
We were able to show that our tetrameric StrepDARPidin is capable of binding different EpCAM-positive cancer cell lineages, which already is a remarkable result. Additionally, we wanted to increase the local concentration of StrepDARPidin molecules and also integrate the activation of the innate immune system for enhancing our CancerSURF. Bacterial surface proteins, such as Flagellin, activate TLR5 (toll-like receptor 5) on dendritic cells, leading to a stimulation of T helper cells. Therefore, employing bacterial flagellar filaments not only drastically increases the amount of StrepDARPidin present on the cancer cell surface but also leads to a stimulation of the inflammatory immune response (Leigh et al. 2014) which creates an anti-tumor environment (Tarahomjoo et al. 2013) .
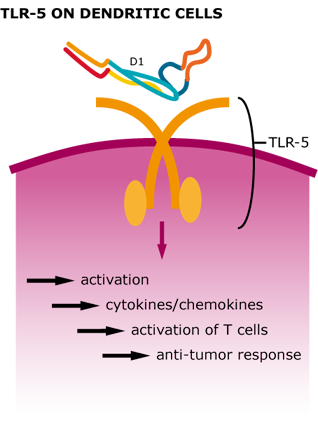 Figure 5: Cellular response after TLR-5 activation by Flagellin.
Figure 5: Cellular response after TLR-5 activation by Flagellin.
Coupling StrepDARPidin to engineered Hag-D2-Strep
As described previously, enhancing the local concentration of DARPin molecules is crucial to allow a rapid and efficient targeting of cancer cells. Therefore, B. subtilis Flagellin was engineered carrying an insertion in the D1-domain replacing the small native loop (see SilverSURF for detailed information) with the S. typhimurium D2-domain and a Strep-Tag. The Hag-D2-Strep (BBa_K1329003) was used for the generation of modified B. subtilis strains as well as overexpression in E. coli BL21 (DE3) cells (Figure 6A). As Flagellin tends to form polymers when overexpressed (a natural feature necessary to build up the filament), it was co-purified with its cognate chaperone FliS, forming a stable and stoichiometric complex on a size-exclusion column (Figure 6A). The fractions of the main peak were pooled and concentrated to a final volume of 500 µL for further analysis.
 Figure 6: Purification of Hag-D2-Strep and binding
assays with StrepDARPidin. (A) Size-exclusion
chromatogram of Hag-D2-Strep
in complex with its chaperone FliS. Upper SDS-PAGE: Ni-NTA purification.
M: marker, L: lysate, FT: flow through, W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions
taken from size-exclusion fractions. (B) Streptavidin- and GST-interaction assay using purified
Hag-D2-Strep/FliS
and Hag-Strep/FliS. (1) Strep-beads without protein (contaminant protein at 30 kDa). (2) Strep-beads with Hag-Strep/FliS immobilized. (3)
Strep-beads with Hag-D2-Strep/FliS.
(4) GST-beads with Hag-Strep/FliS. (5) GST-beads with Hag-D2-Strep/FliS.
(C) Interaction of StrepDARPidin and
Hag-D2-Strep/FliS
visualized by competition assays. Soluble fractions:
(1) Hag-Strep/FliS. (2) Hag-D2-Strep/FliS.
(3) Hag-Strep/FliS and StrepDARPidin. (4)
Hag-D2-Strep/FliS and
StrepDARPidin. (5)
StrepDARPidin. Insoluble fractions: (6) Hag-Strep/FliS and
StrepDARPidin (1:5 dilution). (7)
Hag-D2-Strep/FliS and
StrepDARPidin (1:5 dilution).(8) Hag-Strep/FliS and
StrepDARPidin (1:1 dilution).
(9) Hag-D2-Strep/FliS
and StrepDARPidin (1:1 dilution).
Figure 6: Purification of Hag-D2-Strep and binding
assays with StrepDARPidin. (A) Size-exclusion
chromatogram of Hag-D2-Strep
in complex with its chaperone FliS. Upper SDS-PAGE: Ni-NTA purification.
M: marker, L: lysate, FT: flow through, W: wash, E: elution. Lower SDS-PAGE: Samples of peak fractions
taken from size-exclusion fractions. (B) Streptavidin- and GST-interaction assay using purified
Hag-D2-Strep/FliS
and Hag-Strep/FliS. (1) Strep-beads without protein (contaminant protein at 30 kDa). (2) Strep-beads with Hag-Strep/FliS immobilized. (3)
Strep-beads with Hag-D2-Strep/FliS.
(4) GST-beads with Hag-Strep/FliS. (5) GST-beads with Hag-D2-Strep/FliS.
(C) Interaction of StrepDARPidin and
Hag-D2-Strep/FliS
visualized by competition assays. Soluble fractions:
(1) Hag-Strep/FliS. (2) Hag-D2-Strep/FliS.
(3) Hag-Strep/FliS and StrepDARPidin. (4)
Hag-D2-Strep/FliS and
StrepDARPidin. (5)
StrepDARPidin. Insoluble fractions: (6) Hag-Strep/FliS and
StrepDARPidin (1:5 dilution). (7)
Hag-D2-Strep/FliS and
StrepDARPidin (1:5 dilution).(8) Hag-Strep/FliS and
StrepDARPidin (1:1 dilution).
(9) Hag-D2-Strep/FliS
and StrepDARPidin (1:1 dilution).
To investigate whether the purified Hag-D2-Strep is able to interact with Streptavidin in vitro prior to labeling intact filaments from B. subtilis flagella, we used Streptavidin-pulldown assays to confirm their functionality. For this we immobilized purified Hag-D2-Strep and Hag-Strep on Streptavidin- and GST-beads (Figure 6B). Both engineered variants of Flagellin were able to bind to Streptavidin-beads but not GST and thus confirmed that the Strep-Tag is accessible and functional (Figure 5B, lanes 2 and 3).
Furthermore, we wanted to know if our functional StrepDARPidin is capable of binding to the engineered Flagellin variants. This was the last and crucial step that had to be verified before the docking of StrepDARPidin to the flagellar filaments could be performed. Purified proteins were mixed in different ratios and precipitation was observed. Samples from the supernatant (soluble fraction) and the pellet (insoluble fraction) were loaded on a SDS-PAGE showing that the pellet contained a huge amount of StrepDARPidin and the Flagellin variants (Figure 5C). We assumed that the strong interaction of Streptavidin with the Strep-Tag leads to an aggregation of Hag-Strep variants followed by precipitation (Figure 5C, lanes 6-9).
The D2-Strep variant of Flagellin was also used for replacing the native Flagellin in B. subtilis as described before (see SilverSURF for detailed information) in order to generate a strain exposing the Strep-Tag on the surface of the flagellar filament. In vitro assays confirmed that the Hag-D2-Strep was stable and functional but we had no information about the incorporation of the engineered Flagellin variant into the flagellar filament in vivo. Therefore, we inoculated swimming plates (containing 0.3 % agar) and swarming plates (containing 0.7 % agar) with B. subtilis wild type and D2-Strep cells. The mutant strain showed only slightly slower spreading on both plates, indicating that both single cellular and multi cellular movement is not strongly affected (Figure 6E, F). Additionally, we used negative staining transmission electron microscopy and were able to show that D2-Strep cells are as much flagellated as the wild type (Figure 6A, B). Finally, we can state that our engineered Flagellin variant D2-Strep is fully functional and incorporated into intact flagellar filaments. The last step, immobilizing StrepDARPidin on purified filaments, is currently finalized in the laboratory.
 Figure 7: Electron micrographs of uranyl-acetate stained B. subtilis cells. (A) Whole cell of wild type strain
3610 (B) Whole cell of Hag-D2-Strep strain. Scale bar represents 1 μm.
(C) Magnification of flagellar filaments from 3610 wild type strain. Scale bar represents 100 nm (D) Magnification of flagellar filaments
from Hag-D2-Strepstrain. Scale bar represents 300 nm. (E) Swarming assay of both B. subtilis strains.
(F) Swimming assay of both B. subtilis strains.
Figure 7: Electron micrographs of uranyl-acetate stained B. subtilis cells. (A) Whole cell of wild type strain
3610 (B) Whole cell of Hag-D2-Strep strain. Scale bar represents 1 μm.
(C) Magnification of flagellar filaments from 3610 wild type strain. Scale bar represents 100 nm (D) Magnification of flagellar filaments
from Hag-D2-Strepstrain. Scale bar represents 300 nm. (E) Swarming assay of both B. subtilis strains.
(F) Swimming assay of both B. subtilis strains.
Future perspectives in therapy and diagnostics
The CancerSURF is a novel, modular and efficient method to target different cancer cell lineages and opens the gate for new applications in cancer treatment. The flagellar filament serves as a huge scaffold that can be loaded with different agents of ones own choice. By fusing the tetrameric Streptavidin to small molecules such as DARPins, a wide range of different applications is imaginable. Combining e.g. StrepDARPidin and fluorescent proteins fused to Streptavidin, cancer cells could be visualized during clinical surgeries. Cytotoxic agents in combination with StrepDARPidin could directly lead to cancer cell lysis without causing collateral damage to the surrounding tissues making StrepDARPidin an imaginable antibody alternative for both future diagnostic and therapeutic perspectives.
 Figure 8: Model for possible future applications in lung cancer treatment.
Figure 8: Model for possible future applications in lung cancer treatment.
One of the strongest interactions established by biological molecules is the interaction between Streptavidin and biotin, a cofactor of carboxylases. The property of Streptavidin to tightly bind substrates in combination with engineered Strep-Tags (peptide-tag) has been utilized over the years for several approaches, e.g. biochemical and immunohistochemical methods for protein purification and immunofluorescent stainings.
In 2006, the iGEM team from Harvard used Streptavidin to stain the outer membrane of E. coli cells. By placing the Streptavidin coding sequence downstream of a membrane targeting signal sequence and outer membrane protein under control of an IPTG inducible promoter, the heterologous production in E. coli was achieved to selectively stain the outer membrane by biotinylated fluorophores.
Our aim was to use the extremely tight interaction of Streptavidin and the engineered Strep-TagII, which binds to Streptavidin in the low pico molar range, in combination with DARPin to selectively target epithelial-derived cancer cells and amplify the interaction by our Strep-tagged flagellar filament scaffold. Therefore, we designed a codon-optimized Streptavidin/DARPin chimeric gene (StrepDARPidin) for the heterologous production in E. coli. This chimeric gene enables the efficient production of StrepDARPidin in E. coli and may also serve as a template for the codon optimized Streptavidin gene for future iGEM teams, thereby improving the existing Streptavidin biobrick gene in the registry. Additionally, we established a refolding protocol for the purification of StrepDARPidin and verified its functionality and selectivity by an immunofluorescent analysis of targeted cancer cells and Flagellin-strep binding assays, thereby improving the sole Streptavidin by adding the EpCAM binding property to the protein.
Al-Khodor S, Price CT, Kalia A, Abu Kwaik Y (2010). Ankyrin-repeat containing proteins of microbes: a conserved structure with functional diversity.Trends Microbiol. 18, 132–139 10.1016
Howarth M, TingAY (2008). Imaging proteins in live mammalian cells with biotin ligase and monovalent Streptavidin. Nature Protocols 3, 534 - 545
Leigh ND, Guanglin Bian, Xilai Ding, Hong Liu, Semra Aygun-Sunar, Lyudmila G. Burdelya, Andrei V. Gudkov, Xuefang Cao (2014). A Flagellin-Derived Toll-Like Receptor 5 Agonist Stimulates Cytotoxic Lymphocyte-Mediated Tumor Immunity. PLoS ONE 9(1): e85587
Maaser K, Borlak J (2008). A genome-wide expression analysis identifies a network of EpCAM-induced cell cycle regulators.British Journal of Cancer 99,1635 – 1643
Stefan N, Martin-Killias P, Wyss-Stoeckle (2011). DARPins Recognizing the Tumor-Associated Antigen EpCAM Selected by Phage and Ribosome Display and Engineered for Multivalency. J. Mol. Biol. (2011) 413, 826–843
Stumpp MT, Binz HK, Amstutz P. (2008). DARPins a new generation of protein therapeutics.Drug Discov. Today. Aug. 13(15-16):695-701.
Tarahomjoo S (2013). Utilizing bacterial flagellins against infectious diseases and cancers. Antonie van Leeuwenhoek 105:275–288
Went P, Vasei M, Bubendorf L (2006). Frequent high-level expression of the Immunotherapeutic targetEp-CAM in colon, stomach, prostate and lung cancers. British Journal of Cancer 94, 128 – 135
 "
"
