Team:SUSTC-Shenzhen/Safety
From 2014.igem.org
(→Carefully designed gRNA to decrease off-target effect) |
|||
| (8 intermediate revisions not shown) | |||
| Line 9: | Line 9: | ||
{{SUSTC-Shenzhen/main-content-begin}} | {{SUSTC-Shenzhen/main-content-begin}} | ||
| - | === | + | =Safety Concerns About the Project= |
| + | ==Control the expression of Cas9== | ||
| + | Though CRISPR/Cas genome editing techniques have high specificity due to the gRNA, it still has off-target effect. Chronic expression of Cas9 may amplify such effect and lead to host genome mutagenesis and chromosomal disorders, cytotoxicity, genotoxicity, or oncogenesis. Thus, we want to improve our genetic circuit to reduce the possible chronic side effect while retain its function. Our solution is to utilize chemical inducible system to switch on Cas9 gene when needed and switch it off when not needed. Here, we put the stably transfected Cas9 under the control of Tet-On 3G system, which is the third generation of tetracyclin inducible gene expression systems developed for mammalian cells (Clontech). Target cells that express the Tet-On 3G transactivator protein and contain a gene of interest (GOI) under the control of a TRE3G promoter (PTRE3G) will highly express the GOI, when cultured in the presence of doxycycline (Dox), a synthetic tetracycline derivative. Figure 1 illustrates the mechanism of Tet-ON 3G system (Clontech). | ||
| - | + | <center>{{SUSTC-Image|wiki/images/8/82/SUSTC-Shenzhen-Project-Tet-On.png}}</center> | |
| + | <center>Figure 1 Mechanism of Tet-ON 3G operon</center> | ||
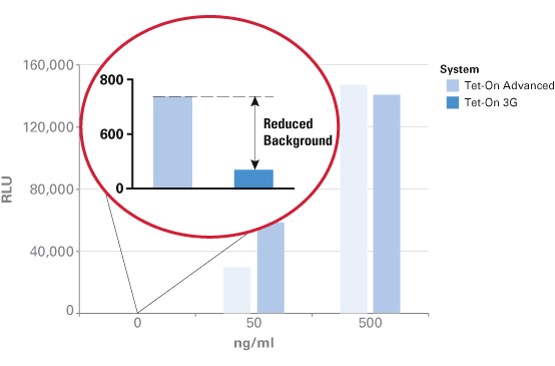
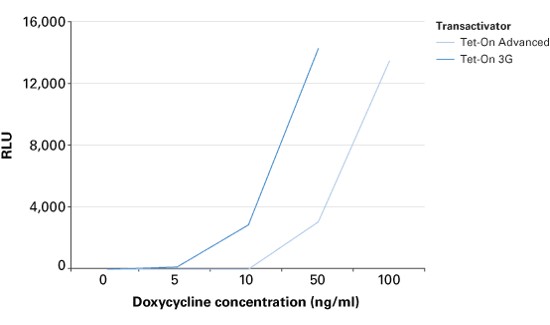
| + | The TRE3G promoter has very low background expression level when Dox is absent. It is the lowest we have found for mammalian inducible system. This greatly reduces potential risks of off-target effect of CRISPR/Cas system (Figure 2). Furthermore, the Dox concentration required for the induction of Tet-On Systems are far below cytotoxic levels for either cell culture or transgenic studies which also reduces the usage of antibiotics (Figure 3). Such on-demand expression system with minimum leaking will greatly alleviate the potential safety risk of the CRISPR/Cas and enable the optimizing of the Cas9 expression level and timing in the potential clinical application. So it is ideal to use Tet-ON 3G as a mammalian gene expression controller for this project. | ||
| + | |||
| + | <center>{{SUSTC-Image|wiki/images/b/b6/SUSTC-Shenzhen-Project-TetON-low_background.jpg}}</center> | ||
| + | <center>Figure 2. Low background expression of Tet-On 3G system</center> | ||
| - | + | <center>{{SUSTC-Image|wiki/images/7/70/SUSTC-Shenzhen-Project-TetOn-high_sensitivity.jpg}}</center> | |
| + | <center>Figure 3 High sensitivity of Tet-On 3G system </center> | ||
| + | ==Control the gRNA expression by A-B toxin-based shuttle== | ||
| + | To further improve the safety of our system, we want to control the timing and location of the expression of gRNA. Unlike many other recent researches which introduce gRNA together with Cas9, we introduce gRNA by using A-B toxin-based shuttle. Even Cas9 expression is accidentally turned on, Cas9 won’t work without gRNA. As the plasmid encoding gRNA is only transiently transfected into the cell, which can’t replicate during cell replication and will lost 2-3 days after transfection in typical cell culture. In addition, it will be readily targeted to specific cell type by conjugating either ligand or antibody specifically for the cell type. This will further reduce the off-target effect of the system. | ||
| - | + | ==Carefully designed gRNA to decrease off-target effect== | |
| + | The gRNA we designed has been matched to human genome and has few off-target sites. | ||
| - | + | <html> | |
| - | + | <a href="/Team:SUSTC-Shenzhen/gRNA_Design" class="btn btn-success btn-lg" style="color:#fff">See how we optimized our gRNA design to reduce off-target effect</a> | |
| - | + | </html> | |
| - | + | ||
| - | + | ||
| - | = | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 03:29, 18 October 2014
Safety
Doing research with responsibility
Contents |
Safety Concerns About the Project
Control the expression of Cas9
Though CRISPR/Cas genome editing techniques have high specificity due to the gRNA, it still has off-target effect. Chronic expression of Cas9 may amplify such effect and lead to host genome mutagenesis and chromosomal disorders, cytotoxicity, genotoxicity, or oncogenesis. Thus, we want to improve our genetic circuit to reduce the possible chronic side effect while retain its function. Our solution is to utilize chemical inducible system to switch on Cas9 gene when needed and switch it off when not needed. Here, we put the stably transfected Cas9 under the control of Tet-On 3G system, which is the third generation of tetracyclin inducible gene expression systems developed for mammalian cells (Clontech). Target cells that express the Tet-On 3G transactivator protein and contain a gene of interest (GOI) under the control of a TRE3G promoter (PTRE3G) will highly express the GOI, when cultured in the presence of doxycycline (Dox), a synthetic tetracycline derivative. Figure 1 illustrates the mechanism of Tet-ON 3G system (Clontech).
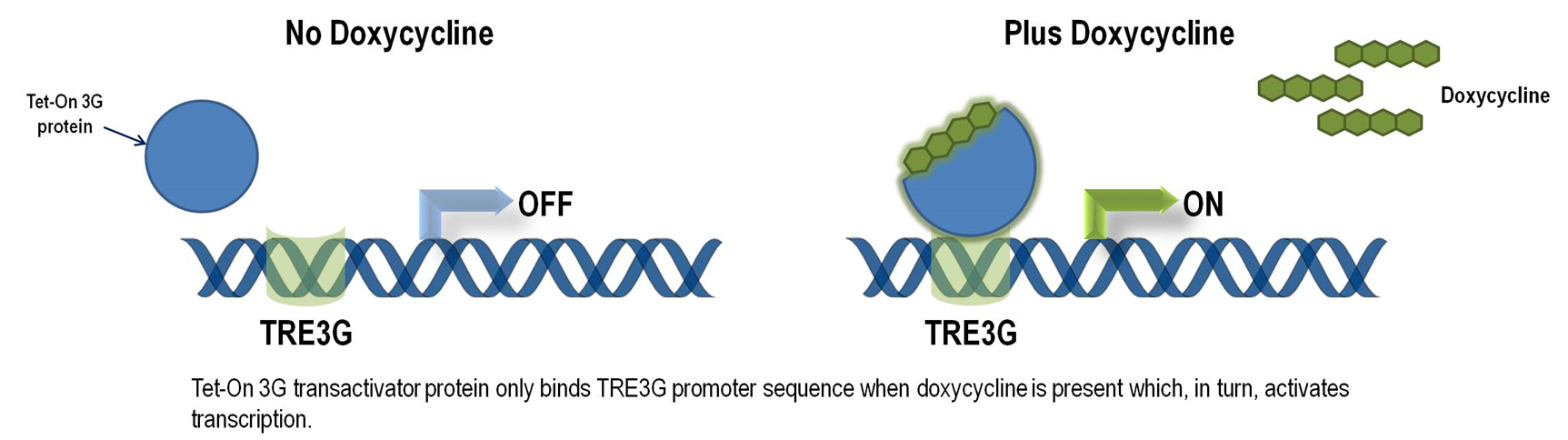
The TRE3G promoter has very low background expression level when Dox is absent. It is the lowest we have found for mammalian inducible system. This greatly reduces potential risks of off-target effect of CRISPR/Cas system (Figure 2). Furthermore, the Dox concentration required for the induction of Tet-On Systems are far below cytotoxic levels for either cell culture or transgenic studies which also reduces the usage of antibiotics (Figure 3). Such on-demand expression system with minimum leaking will greatly alleviate the potential safety risk of the CRISPR/Cas and enable the optimizing of the Cas9 expression level and timing in the potential clinical application. So it is ideal to use Tet-ON 3G as a mammalian gene expression controller for this project.
Control the gRNA expression by A-B toxin-based shuttle
To further improve the safety of our system, we want to control the timing and location of the expression of gRNA. Unlike many other recent researches which introduce gRNA together with Cas9, we introduce gRNA by using A-B toxin-based shuttle. Even Cas9 expression is accidentally turned on, Cas9 won’t work without gRNA. As the plasmid encoding gRNA is only transiently transfected into the cell, which can’t replicate during cell replication and will lost 2-3 days after transfection in typical cell culture. In addition, it will be readily targeted to specific cell type by conjugating either ligand or antibody specifically for the cell type. This will further reduce the off-target effect of the system.
Carefully designed gRNA to decrease off-target effect
The gRNA we designed has been matched to human genome and has few off-target sites.
See how we optimized our gRNA design to reduce off-target effect
 "
"