Team:KAIT Japan/Project
From 2014.igem.org
(Difference between revisions)
| (23 intermediate revisions not shown) | |||
| Line 53: | Line 53: | ||
| style="width:30% style="vertical-align:top" | | | style="width:30% style="vertical-align:top" | | ||
| - | < | + | =<font size="7">Project</font>= |
<br> | <br> | ||
| - | + | =='''<font size="5">Back graund</font>'''== | |
| - | '''<font size=" | + | <font size="4"> |
| + | ・The secondary immune response<br> | ||
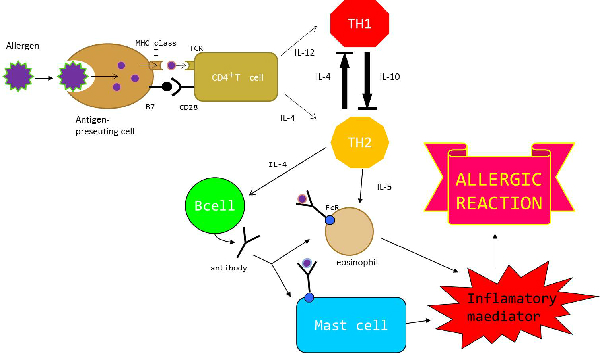
| + | As a person's immune system encounters foreign substances (antigens), the components of acquired immunity learn the best way to attack each antigen and develop a memory for that antigen. | ||
| + | The immune system is turned on when the antigen presenting cell (APC) such as the dendritic cell is taken in by an endocytosis. | ||
| + | The antigen taken in is broken down to peptide by lysosome. | ||
| + | The antigenic peptides form a complx when MHC class Ⅱ molecules composed in endoplasmic reticulum and are carried to the cell surface. The T cell Receptor (TcR) of the T cell (CD4 cell) combines with this complex. This becomes the first signal. | ||
| + | Furthermore, CD28 of the CD4 cell combines with B4 of the APC.to be the supporting signal; CD4 cell is activated by these two signals. | ||
| + | The activated T cell_(CD4+ cell) differentiates to Th2 cell by stimulation of interleukin-4(IL-4) proteins or to Th1 cell by IL-12,respectively. | ||
| + | Th1 participates in cell-mediated innunity to produces IFN-γand IL-2 and activates a macrophage and a killer cell.Th2 ,on the other hand,participates in humoral immunity-to produces IL-4, IL-5,_IL-10 and activates B cell and a mast cell. | ||
| + | In this way, Th1 and Th2 play important roles in immune reaction. | ||
<br> | <br> | ||
<br> | <br> | ||
| - | + | ・Interaction between Th1 and Th2<br> | |
| + | Depending on pathogens and stimulation of immunitys,a kind of the immune response is decided either the Th1 predominance or the Th2 predominance. | ||
| + | When a virus invades the body, differentiation to Th1 is promoted by Il-12 produced by APC, and Th1 produce IFN-γ for phylaxis. At the same time, Th2 is controlled by IFN-γ, and the production of cytokine promoting mast cells and eosinophil is also controlled . On the other hand, when an antigen invades body, differentiation to Th2 is promoted by IL-4 . Th2 produces IL-4, and activates B cells to produce an antibody (IgE,IgG). Moreover, Th2 produces IL-5 and promotes eosinophil work. At the same time, Th1 is controlled by IL-4, and the production of cytokine promoting the function of macrophages is also controlled. | ||
| + | In this way, Th1 and Th2 usually keep the mutual balance to control immune response. (Th1/Th2 balance) | ||
| + | When this balance is lost and declined to the Th2 predominance, IL-5 is secreted excessively from Th2, and eosonophil and mast cells are activated. Subsequently, that causes excessive secretion of inflammatory materials such as histamine or leukotriene when an antigen is connected to those activated cells having antibody bound to through Fc receptor (FcR). This inflammatory material and antibody produced excessively are related to allergic reactions. | ||
| + | The allergic reactions are classified from type Ⅰto Ⅳ;these reactions can occur independently,or multiple types may be taking place at the same time. | ||
| + | Above all, type Ⅰ reactions is called immediate hypersensitivity, and the onset appears in a very short time. | ||
| + | _Examples of this type include bronchial asthma and atopic dermatitis | ||
| + | There are the people suffering by these allergic diseases. | ||
| + | Therefore, we thought that keeping the Th1/Th2 balance by increasing IL-12 which participates in Th1 cytodifferentiation can restrain allergic reactions. | ||
| + | </font> | ||
<br> | <br> | ||
| + | [[File:New ダブルチャージ.jpg]] | ||
| + | |||
<br> | <br> | ||
| - | |||
<br> | <br> | ||
| + | |||
| + | =='''<font size="5">Link Light Assay</font>'''== | ||
| + | <font size="4"> | ||
| + | A variety of protein interaction analyses have been developed. Linklight Assay is a novel method based on an inactive permuted protein which can be activated by a protease to generate stable and sensitive signals for monitoring protein interaction. | ||
| + | Linklight Assay consists of two components: A protein A fused to a Tobacco Etch Virus (TEV) protease and a protein B fused to a permuted luciferase (pLuc) containing a TEV protease cleavage sequence. An inactive pLuc can be created by breaking a luciferase into two fragments, rearranging the fragment order as the N-terminal fragment moved to C-terminus and C-terminal fragment moved to the N-terminus. and connecting them with a TEV protease cleavage sequence. After interaction between protein A and B, inactive pLuc is cleaved, and the cleaved luciferase fragments spontaneously refold to reconsititute an active luciferase. Active luciferase works as reporter. | ||
| + | Linklight Assay provides simple, robust, economic, and sensitive methods to detect protein interaction in live cells with immediate and stable signals. | ||
| + | In this study applied this technology to make IL-5 independent expression system. We will explain our strategy for Ecoli receiving IL5, and for producing IL-12 in Strategy Page. | ||
| + | </font> | ||
<br> | <br> | ||
| - | + | [[File:New ビックカメラ.jpg]] | |
| + | |||
<br> | <br> | ||
<br> | <br> | ||
| - | '''<font size=" | + | |
| + | =='''<font size="5">HRV protease</font>'''== | ||
| + | <font size="4"> | ||
| + | TEV protease is a highly sequence-specific cysteine protease from Tobacco Etch Virus (TEV). Due to its high sequence specificity, it is frequently used for the controlled cleavage of fusion proteins. | ||
| + | Human Rhinovirus (HRV) 14 3C protease is a cysteine protease used to remove fusion from proteins with the HRV14 3C cleavage sequence, like TEV protease. HRV14 3C protease has also high specific recognition sequence to Leu-Glu-Val-Leu-Phe-Gln-↓-Gly-Pro as the substrate. The 22 kDa protease get its optimal activity at 4 ℃, but also shows a highiy cleavage rate at 37℃. | ||
| + | HRV14 3C protease is very similar to TEV protease. So, we thought that HRV14 3C protease can cleave protease cleavage sequence which connects IL5 receptor and AraC in the same way TEV protease cleavage protein B fused to a pLuc containing a TEV protease cleavage sequence in linklight assay. | ||
| + | </font> | ||
| + | |||
<br> | <br> | ||
<br> | <br> | ||
| - | '''<font size=" | + | |
| + | =='''<font size="5">Methodology</font>'''== | ||
| + | <font size="4"> | ||
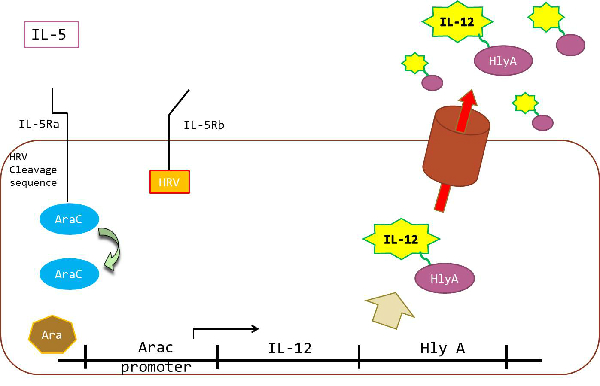
| + | Strategy for ecoli to receive IL5, and to produces IL-12. | ||
| + | Applying Linklight assay, our goal is to create an ecoli which receives IL-5,and produces IL-12. | ||
| + | IL-5 is an interleukin produced by T helper 2 cells, mast cells and eosinophils. Through binding to the IL-5 receptor, IL-5 stimulates B cells growth and increases immunoglobulin secretion. It is also a key mediator in eosinophils activation. | ||
| + | IL-5receptor consists of a heterodimer of an alpha subunit and a beta subunit. The IL-5 receptor alpha (IL-5Ra) is specific to IL-5. The beta chain (IL-5Rb) is the common beta chain of receptors for IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF). After IL-5 binds to IL-5Ra, IL-5Rb meets IL-5 and form heterodimer with alpha subunit. If IL-5 does not bind to IL-5Ra, IL-5Rb cannot form heterodimer with alpha subunit.<br> | ||
| + | |||
| + | The method applied for Ecoli:to receive IL-5and to produce IL-12 is as follows: | ||
| + | Fusing IL-5Ra and IL-5Rb with AraC which contains a HRV protease cleavage sequence and HRV protease respectively. If heterodimer of IL-5Ra and IL-5Rb are formed by IL-5, AraC containing cleavage sequence and HRV are forced into close proximity. HRV protease recognizes cleavage sequence connecting IL5Ra and AraC and cleaves it. AraC is released from IL5Ra by HRV protease. After released from IL5Ra, AraC regulates expression of IL12 coding gene downstream of AraC promoter. When arabinose is present, AraC acts an activator, and activates expression of IL12.HlyA C-terminal signal is a protein secrete tag. So IL12 which has HlyA secrete tag are secreted outside an ecoli. In the lour experiment, we use GFP instead of IL12 to simplify result. This strategy enable for ecoli to receive IL5 and produce IL12. | ||
| + | </font> | ||
| + | <br> | ||
| + | [[File:New 臨界ブラ木.jpg]] | ||
Latest revision as of 09:42, 17 October 2014
|
|
|
|
|
|
|
|
|
 "
"