Team:Freiburg/Content/Project/The light system
From 2014.igem.org
| Line 48: | Line 48: | ||
</a> | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Principle of the blue light inducible expression system based on the LOV2 domain from the common oat <em class="highlight-kursiv">Avena sativa</em>.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 69: | Line 69: | ||
</a> | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Principle of the red light inducible expression system based on phytochrome B and (PhyB) and the phytochrome-interacting factor 6 (PIF6) from the model organism <em class="highlight-kursiv">Arabidopsis thaliana</em>.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
Revision as of 16:31, 17 October 2014
The Light System
Optogenetics In Mammalian Gene Expression
The term "Optogenetics" describes the use of genetically encoded, light-sensing proteins to modulate cellular function or even organismal behavior with high spatio-temporal resolution. In the past few years a large number of versatile light-controlled tools and devices have been developed in all kinds of species, reaching from bacteria (1) to mammalian cells (2). The introduction of microbial melanopsin into neurons (3) was the first optogenetic approach in mammalian cells and constitutes a milestone in neuronal research (4). More recently, light-regulated tools have been successfully applied to control diverse signaling processes and gene expression in mammalian cells (2,5).
Light inducible gene expression systems follow the yeast two hybrid principle, using photoreceptors, which allow a number of species to respond to environmental light conditions, as building blocks. In synthetically designed systems, light mediates the recruitment of a transcriptional activation domain to the DNA site. Therefore, either photoreceptors and their light-dependent specific interaction partners are fused to a transactivation domain or to a DNA binding domain, respectively, or light-induced homo-dimerization of photoreceptors is exploited to reconstitute a DNA binding domain. To date, spatio-temporal control over mammalian gene expression is provided by optogenetic gene switches, which are responsive to three distinct wavelength ranges in the blue, red and UV (6).
The AcCELLerator Light Systems
In order to choose the light system best suited for our experiments, we considered advantages and disadvantages of all three, such as possible toxic side effects, ease of handling and induction rate. UVB light inducible gene switches show high induction rates, but UV light has a toxic effect on cells, and was therefore excluded (7). In contrast, for the red and blue light inducible systems, the light exposure has minimal toxic side effects.
The red light system has the additional advantage of high tissue penetration, but on the other hand requires the addition of the small molecule compound PCB to work (8). Both the red and blue light systems are very sensitive to unintentional activation by room light and therefore require special care during handling (7). Since this problem can be overcome by working under safe green light conditions, we considered both, blue and red light-inducible systems, as suited for our application.
LOV2 Based Blue Light Responsive System
The blue light expression system used for the AcCELLarator capitalizes on the second LOV (Light-Oxygen-Voltage) domain of the protein phototropin of Avena sativa (AsLOV2). LOV domains are small photosensory peptides with up to 125 residues and are used by a large variety of higher plants, microalgae, fungi and bacteria to sense environmental conditions. LOV domains, such as the AsLOV2 domain, have been successfully employed in numerous designs for optogenetic tools. AsLOV2 is N- and C-terminally flanked by α helices, reffered to as the Aα and Jα Helix, respectively.
In addition to the LOV2 domain, there are several other parts necessary for light induced expression of target genes. They can be separated into two main modules:
One includes the previously mentioned LOV2 domain that is fused to a Gal4-DNA binding domain (Gal4DBD). This part is constantly located at a specific DNA sequence, the Gal4-upstream activator sequence (Gal4UAS) nearby the promotor region of a target gene.
The second part consists of an Erbin PDZ domain (ePDZ) that is fused to a VP-16 domain. The VP-16 domain can act as a transcriptional activator which recruits DNA polymerase to the target gene.
The interaction between ePDZ and the Jα helix of LOV2 is the key process of the system: While in the dark, Jα chain is not exposed, therefore, the ePDZ-VP-16 domain can not be recruited and no gene expression is detectable. Upon illumination, the Jα chain of the LOV2-domain becomes accessible enabling the second part of the light system, epdZ fused toVP16, to bind to the Jα chain. VP-16 recruits DNA polymerase to the target gene thereby leading to a magnification of gene expression.

Principle of the blue light inducible expression system based on the LOV2 domain from the common oat Avena sativa.
Red/Far Red Light Responsive System
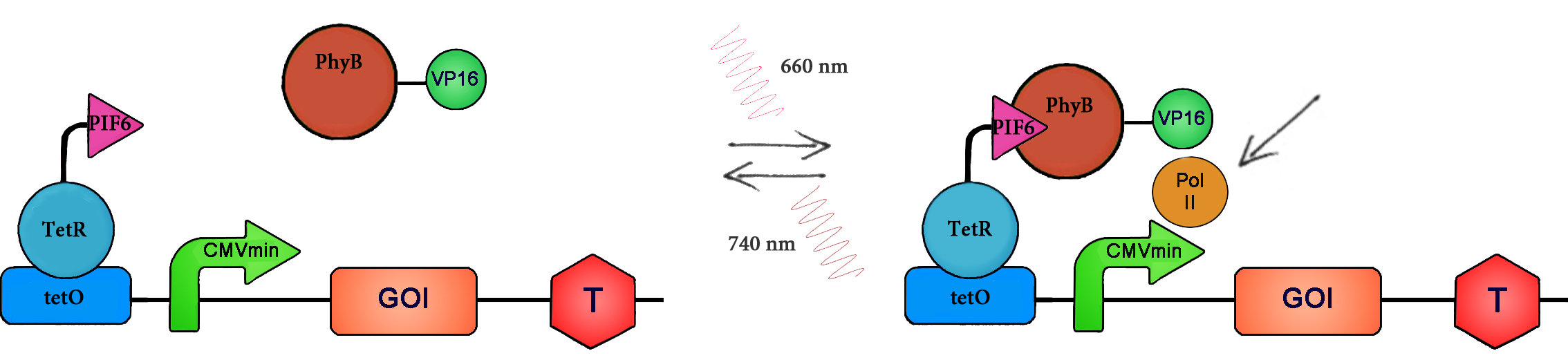
Red light triggered gene expression is based on the Arabidopsis thaliana proteins phytochrome B (PhyB) and the phytochrome-interacting factor 6 (PIF6). PhyB is a photoreceptor with a N-terminal photosensory domain, which autoligates its chromophore phytochromobilin. There are two different states of PhyB: Under red light (660nm), isomerisation of the chromophore results in the constitution of the active PFR form of the protein, which can bind to PIF6. Far red light illumination leads to reversion back into the inactive PR form and to dissociation of PhyB and PIF6. To capitalize on this light dependent interaction, the N-terminal part of PhyB has been fused to the herpes simplex virus derived transactivation domain VP16. Further, the N-terminal domain of PIF6 was engineered to bind to the tetR-specific operator tetO upstream of the minimal human cytomegalovirus immediate-early promoter PhCMVmin by fusion to tetR, which binds PIF6 to the DNA.
All in all, both light systems are activated by photoexcitation: The LOV-system turns to the „ON“ state, leads to the recruitment and binding of ePDZ to the Jα helix and to induction of target gene expression. Under dark conditions the system turns back into the „OFF“ state where transcription is terminated (9). In the red light system, absorption of a red photon (660nm) results in the recruitment of PhyB and the fused transcriptional activation domain to the promoter, which subsequently initiates transcription. The system can be immediately shut off, by illumination with far red light (740nm)(8).
In our application, The AcCELLerator, the target gene that is selectively induced by photoexcitation is mCAT-1, a murine cationic amino acid transporter. To explore the possibilities by using this transporter, visit the next side and find out, why mCAT-1 is also termed as “receptor”.Read More about mCAT-1!
References
- Levskaya A, Chevalier AA, Tabor JJ, Simpson ZB, Lavery LA, Levy M, Davidson EA, Scouras A, Ellington AD, Marcotte EM, Voigt CA. Engineering Escherichla coli to see light. Nature. 2005;438:441–44
- Muller, K., and Weber, W. (2013). Optogenetic tools for mammalian systems. Mol Biosyst 9, 596-608.
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A.2003;100:13940–13945
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268
- Pathak, G.P., Vrana, J.D., and Tucker, C.L. (2013) Optogenetic control of cell function using engineered photoreceptors. Biol Cell 105, 59-72
- Muller, K., Engesser, R., Schulz, S., Steinberg, T., Tomakidi, P., Weber, C.C., Ulm, R., Timmer, J., Zurbriggen, M.D., and Weber, W. (2013b). Multi-chromatic control of mammalian gene expression and signaling. Nucleic Acids Res 41, e124.
- Müller K, Naumann S, Weber W, and Zurbriggen M.D. Optogenetics for gene expression in mammalian cells. Biol Chem. 2014 Aug 2. pii: /j/bchm.just-accepted/hsz-2014-0199/hsz-2014-0199.xml. doi: 10.1515/hsz-2014-0199
- Muller,K., Engesser,R., Metzger,S., Schulz,S., Kampf,M.M., Busacker,M., Steinberg,T., Tomakidi,P., Ehrbar,M., Nagy,F. et al. (2013) A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res., 41, e77.
- Strickland D, et al. (2012) TULIPs: tunable, light-controlled interacting protein tags for cell biology.LID - 10.1038/nmeth.1904 [doi] Nat Methods
 "
"