Team:KAIT Japan/Project
From 2014.igem.org
(Difference between revisions)
| (2 intermediate revisions not shown) | |||
| Line 53: | Line 53: | ||
| style="width:30% style="vertical-align:top" | | | style="width:30% style="vertical-align:top" | | ||
| - | + | =<font size="7">Project</font>= | |
<br> | <br> | ||
=='''<font size="5">Back graund</font>'''== | =='''<font size="5">Back graund</font>'''== | ||
| - | <font size="4"> | + | <font size="4"> |
| + | ・The secondary immune response<br> | ||
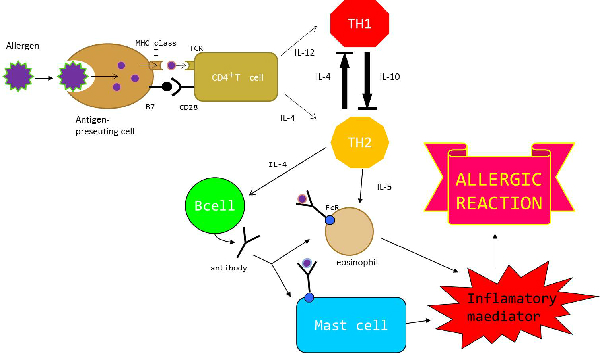
As a person's immune system encounters foreign substances (antigens), the components of acquired immunity learn the best way to attack each antigen and develop a memory for that antigen. | As a person's immune system encounters foreign substances (antigens), the components of acquired immunity learn the best way to attack each antigen and develop a memory for that antigen. | ||
The immune system is turned on when the antigen presenting cell (APC) such as the dendritic cell is taken in by an endocytosis. | The immune system is turned on when the antigen presenting cell (APC) such as the dendritic cell is taken in by an endocytosis. | ||
| Line 65: | Line 66: | ||
Th1 participates in cell-mediated innunity to produces IFN-γand IL-2 and activates a macrophage and a killer cell.Th2 ,on the other hand,participates in humoral immunity-to produces IL-4, IL-5,_IL-10 and activates B cell and a mast cell. | Th1 participates in cell-mediated innunity to produces IFN-γand IL-2 and activates a macrophage and a killer cell.Th2 ,on the other hand,participates in humoral immunity-to produces IL-4, IL-5,_IL-10 and activates B cell and a mast cell. | ||
In this way, Th1 and Th2 play important roles in immune reaction. | In this way, Th1 and Th2 play important roles in immune reaction. | ||
| - | + | <br> | |
| - | + | <br> | |
| - | + | ・Interaction between Th1 and Th2<br> | |
Depending on pathogens and stimulation of immunitys,a kind of the immune response is decided either the Th1 predominance or the Th2 predominance. | Depending on pathogens and stimulation of immunitys,a kind of the immune response is decided either the Th1 predominance or the Th2 predominance. | ||
When a virus invades the body, differentiation to Th1 is promoted by Il-12 produced by APC, and Th1 produce IFN-γ for phylaxis. At the same time, Th2 is controlled by IFN-γ, and the production of cytokine promoting mast cells and eosinophil is also controlled . On the other hand, when an antigen invades body, differentiation to Th2 is promoted by IL-4 . Th2 produces IL-4, and activates B cells to produce an antibody (IgE,IgG). Moreover, Th2 produces IL-5 and promotes eosinophil work. At the same time, Th1 is controlled by IL-4, and the production of cytokine promoting the function of macrophages is also controlled. | When a virus invades the body, differentiation to Th1 is promoted by Il-12 produced by APC, and Th1 produce IFN-γ for phylaxis. At the same time, Th2 is controlled by IFN-γ, and the production of cytokine promoting mast cells and eosinophil is also controlled . On the other hand, when an antigen invades body, differentiation to Th2 is promoted by IL-4 . Th2 produces IL-4, and activates B cells to produce an antibody (IgE,IgG). Moreover, Th2 produces IL-5 and promotes eosinophil work. At the same time, Th1 is controlled by IL-4, and the production of cytokine promoting the function of macrophages is also controlled. | ||
| Line 89: | Line 90: | ||
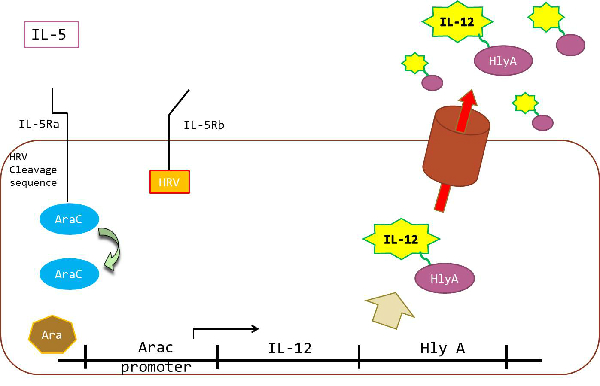
Linklight Assay consists of two components: A protein A fused to a Tobacco Etch Virus (TEV) protease and a protein B fused to a permuted luciferase (pLuc) containing a TEV protease cleavage sequence. An inactive pLuc can be created by breaking a luciferase into two fragments, rearranging the fragment order as the N-terminal fragment moved to C-terminus and C-terminal fragment moved to the N-terminus. and connecting them with a TEV protease cleavage sequence. After interaction between protein A and B, inactive pLuc is cleaved, and the cleaved luciferase fragments spontaneously refold to reconsititute an active luciferase. Active luciferase works as reporter. | Linklight Assay consists of two components: A protein A fused to a Tobacco Etch Virus (TEV) protease and a protein B fused to a permuted luciferase (pLuc) containing a TEV protease cleavage sequence. An inactive pLuc can be created by breaking a luciferase into two fragments, rearranging the fragment order as the N-terminal fragment moved to C-terminus and C-terminal fragment moved to the N-terminus. and connecting them with a TEV protease cleavage sequence. After interaction between protein A and B, inactive pLuc is cleaved, and the cleaved luciferase fragments spontaneously refold to reconsititute an active luciferase. Active luciferase works as reporter. | ||
Linklight Assay provides simple, robust, economic, and sensitive methods to detect protein interaction in live cells with immediate and stable signals. | Linklight Assay provides simple, robust, economic, and sensitive methods to detect protein interaction in live cells with immediate and stable signals. | ||
| - | In this study applied this technology to make IL-5 independent expression system. We will explain our strategy for Ecoli receiving IL5, and for producing IL-12 in Strategy Page | + | In this study applied this technology to make IL-5 independent expression system. We will explain our strategy for Ecoli receiving IL5, and for producing IL-12 in Strategy Page. |
</font> | </font> | ||
<br> | <br> | ||
Latest revision as of 09:42, 17 October 2014
|
|
|
|
|
|
|
|
|
 "
"