Team:SUSTC-Shenzhen/Notebook/Biobricks Characterization
From 2014.igem.org
Zhangysh1995 (Talk | contribs) |
Zhangysh1995 (Talk | contribs) |
||
| Line 10: | Line 10: | ||
='''Scheme'''= | ='''Scheme'''= | ||
| + | At first, we want to characterize plasmid assembled by 3 promoters, 3 RBSs, and 4 chromoprotein (36). Because time limits, we choose 2 promoter, 2 RBSs and 4 chromoprotein (16). In carrying out experiments, we cannot easily differ new constructed plasmid with BBa_E1010 with the self-assembly one. We abandoned BBa_E1010 and do experiments on other chromoproteins. | ||
| + | |||
| + | ='''Results'''= | ||
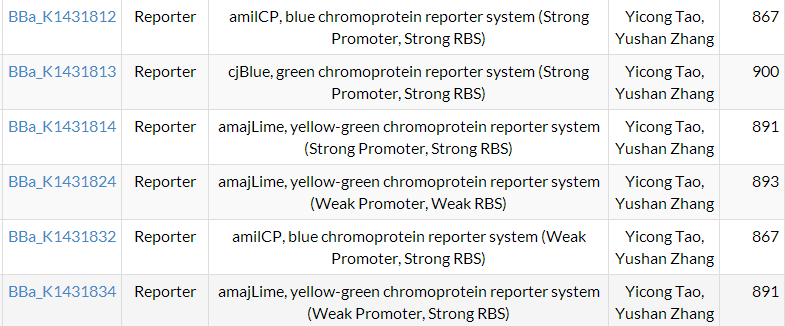
| + | We successfully constructed 8 parts, and they all are characterized. And 6 parts were sent to Registry of Standard Biological Parts. See them [https://2014.igem.org/Team:SUSTC-Shenzhen/Parts | HERE]. | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/e/ef/SUSTC-Shenzhen_Characterization_Parts.png|Parts}} | ||
=='''Procedures'''== | =='''Procedures'''== | ||
| Line 23: | Line 29: | ||
After RBS added, all seven plasmid were cut and ligated with two promoter, J23101 and J23106 respectively. | After RBS added, all seven plasmid were cut and ligated with two promoter, J23101 and J23106 respectively. | ||
==='''Enzyme digestion'''=== | ==='''Enzyme digestion'''=== | ||
| - | + | For plan A | |
| + | {| class=''table'' | ||
| + | ! | ||
| + | !J00 | ||
| + | !J06 | ||
| + | !B31E10 | ||
| + | !B31K916 | ||
| + | !B31K09 | ||
| + | !B34E10 | ||
| + | !B34K916 | ||
| + | !B34K09 | ||
| + | !B34K11 | ||
| + | |- | ||
| + | |EcoRI-HF(μL) | ||
| + | |colspan='9'|1.0 | ||
| + | |- | ||
| + | |XbaI(μL) | ||
| + | |colspan='9'|1.0 | ||
| + | |- | ||
| + | |PstI | ||
| + | |colspan='2'| | ||
| + | |colspan='7'|1.0 | ||
| + | |- | ||
| + | |NcoI | ||
| + | |colspan='2'|1.0 | ||
| + | |colspan='6'| | ||
| + | |1.0 | ||
| + | |- | ||
| + | |Linearized backbone(μL) | ||
| + | |colspan='9'|1.0 | ||
| + | |- | ||
| + | |DNA(μL) | ||
| + | |3.0 | ||
| + | |4.0 | ||
| + | |8.0 | ||
| + | |7.0 | ||
| + | |4.0 | ||
| + | |colspan='4'|5.0 | ||
| + | |- | ||
| + | |10x NEB Buffer 2.1(μL) | ||
| + | |5.0 | ||
| + | |- | ||
| + | |ddH2O(μL) | ||
| + | |39 | ||
| + | |38 | ||
| + | |34 | ||
| + | |35 | ||
| + | |38 | ||
| + | |colspan='4'|37 | ||
| + | |- | ||
| + | |Total(μL) | ||
| + | |colspan='9'|40 | ||
| + | | | ||
| + | } | ||
==='''Ligation'''=== | ==='''Ligation'''=== | ||
To complete construction quickly, we use 3A assembly to achieve plasmid with resistant to chloramphenicol (A) and standard assembly with resistant to Ampicillin (B). | To complete construction quickly, we use 3A assembly to achieve plasmid with resistant to chloramphenicol (A) and standard assembly with resistant to Ampicillin (B). | ||
Revision as of 07:05, 17 October 2014
Notebook
Biobricks Characterization
Contents |
Scheme
At first, we want to characterize plasmid assembled by 3 promoters, 3 RBSs, and 4 chromoprotein (36). Because time limits, we choose 2 promoter, 2 RBSs and 4 chromoprotein (16). In carrying out experiments, we cannot easily differ new constructed plasmid with BBa_E1010 with the self-assembly one. We abandoned BBa_E1010 and do experiments on other chromoproteins.
Results
We successfully constructed 8 parts, and they all are characterized. And 6 parts were sent to Registry of Standard Biological Parts. See them | HERE.
Procedures
- Amplification of Biobricks
- Add RBS
- Add promoter
- Add terminator
Plasmid Construction
9.29 After RBS added, all seven plasmid were cut and ligated with two promoter, J23101 and J23106 respectively.
Enzyme digestion
For plan A
| J00 | J06 | B31E10 | B31K916 | B31K09 | B34E10 | B34K916 | B34K09 | B34K11 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| EcoRI-HF(μL) | 1.0 | |||||||||
| XbaI(μL) | 1.0 | |||||||||
| PstI | 1.0 | |||||||||
| NcoI | 1.0 | 1.0 | ||||||||
| Linearized backbone(μL) | 1.0 | |||||||||
| DNA(μL) | 3.0 | 4.0 | 8.0 | 7.0 | 4.0 | 5.0 | ||||
| 10x NEB Buffer 2.1(μL) | 5.0 | |||||||||
| ddH2O(μL) | 39 | 38 | 34 | 35 | 38 | 37 | ||||
| Total(μL) | 40 |
} LigationTo complete construction quickly, we use 3A assembly to achieve plasmid with resistant to chloramphenicol (A) and standard assembly with resistant to Ampicillin (B). Ligation: In PCR system, 16 to ligate, 65℃ to inactive, and store at 4℃. Transformation
Incubate at 37 CharacterizationReferences
|
||||||||
 "
"