Team:ETH Zurich/labblog/20140825
From 2014.igem.org
(Difference between revisions)
(Created page with "== Diffusion Experiments == ==== Friday, 15th August ==== After having constructed the necessary regulator and sensor plasmids we were able to start first cross talk experiment...") |
|||
| (27 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | <html><article class="mix lab milli carousel" id="diffusionexp" date="20140825"></html> | ||
| + | |||
== Diffusion Experiments == | == Diffusion Experiments == | ||
| - | ==== | + | ==== Monday, 25th August ==== |
| + | |||
| + | Now that we tested the different constructs for leakiness, dose response and cross talk, we are investigating in the diffusion rate of AHL. Therefore we designed a chip with chambers connected by channels of different length (see figure). In one chamber we added a strain containing a regulator and a sensor plasmid, to the second chamber we added AHL or an AHL producer strain. As soon as AHL is diffused to the former chamber it induces sfGFP production on the sensor plasmid. The time it takes until we can measure the sfGFP signal and the length of the channel gives us information about the velocity of AHL diffusion. | ||
| + | |||
| + | [[File:Channelchip.jpg|center|300px|thumb|PDMS millifluidic chip used for diffusion experiments]] | ||
| + | |||
| + | Three main experiment designs were tested and modified: the agar-design, the liquid-design and the beads-design. | ||
| + | * For the agar-design we filled the chamber and channels with LB-agar or in a second attempt with agarose only. Subsequently, we punched a cylindric hole in each chamber. The capacities were filled with regulator/sensor strain culture or AHL/AHL producer strain culture, respectively. After 3 to 4 h at 37 °C we could observe an increase in the sfGFP signal. Since we used comparably high concentration of AHL in our first attempts, we could not detect a delayed sfGFP signal for chambers connected by a longer channel. The experiment will be repeated using lower concentrations. However, conducting the experiment using the agar-design, we encountered a problem: after some hours the LB-agar/agarose started to shrink and dried out. Thus, the agar-design cannot be used without modifications for experiments of longer duration. | ||
| + | * In an attempt we only left a small agar fragment in each channel and filled the rest with liquid medium, so as to decrease chance of it drying out, while avoiding at the same time that bacteria from one chamber reach the other chamber. | ||
| + | * The bead-design consists of an alginate bead for each chamber and liquid medium in between. The beads either contained regulator/sensor-bacteria or AHL. | ||
| + | |||
| + | |||
| + | [[File:ETH_Zurich_Diffusion2.JPG|center|500px|thumb|Diffusion experiment with agar. Click [https://2014.igem.org/Team:ETH_Zurich/Video1 here] to watch the video.]] | ||
| + | |||
| + | [[File:ETH_Zurich_Diffusion3.JPG|center|500px|thumb|Diffusion experiment with liquid. Click [https://2014.igem.org/Team:ETH_Zurich/Video2 here] to watch the video. ]] | ||
| + | |||
| + | [[File:ETH_Zurich_Diffusion4.JPG|center|500px|thumb|Diffusion experiment with beads. Click [https://2014.igem.org/Team:ETH_Zurich/Video3 here] to watch the video.]] | ||
| - | + | {{:Team:ETH_Zurich/tpl/topbutton|green}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | {{:Team:ETH Zurich/tpl/rmbutton|green|diffusionexp}} | |
| - | + | <html> </article></html> | |
| - | <html></article></html> | + | |
Latest revision as of 15:10, 11 October 2014
Diffusion Experiments
Monday, 25th August
Now that we tested the different constructs for leakiness, dose response and cross talk, we are investigating in the diffusion rate of AHL. Therefore we designed a chip with chambers connected by channels of different length (see figure). In one chamber we added a strain containing a regulator and a sensor plasmid, to the second chamber we added AHL or an AHL producer strain. As soon as AHL is diffused to the former chamber it induces sfGFP production on the sensor plasmid. The time it takes until we can measure the sfGFP signal and the length of the channel gives us information about the velocity of AHL diffusion.
Three main experiment designs were tested and modified: the agar-design, the liquid-design and the beads-design.
- For the agar-design we filled the chamber and channels with LB-agar or in a second attempt with agarose only. Subsequently, we punched a cylindric hole in each chamber. The capacities were filled with regulator/sensor strain culture or AHL/AHL producer strain culture, respectively. After 3 to 4 h at 37 °C we could observe an increase in the sfGFP signal. Since we used comparably high concentration of AHL in our first attempts, we could not detect a delayed sfGFP signal for chambers connected by a longer channel. The experiment will be repeated using lower concentrations. However, conducting the experiment using the agar-design, we encountered a problem: after some hours the LB-agar/agarose started to shrink and dried out. Thus, the agar-design cannot be used without modifications for experiments of longer duration.
- In an attempt we only left a small agar fragment in each channel and filled the rest with liquid medium, so as to decrease chance of it drying out, while avoiding at the same time that bacteria from one chamber reach the other chamber.
- The bead-design consists of an alginate bead for each chamber and liquid medium in between. The beads either contained regulator/sensor-bacteria or AHL.

Diffusion experiment with agar. Click here to watch the video.
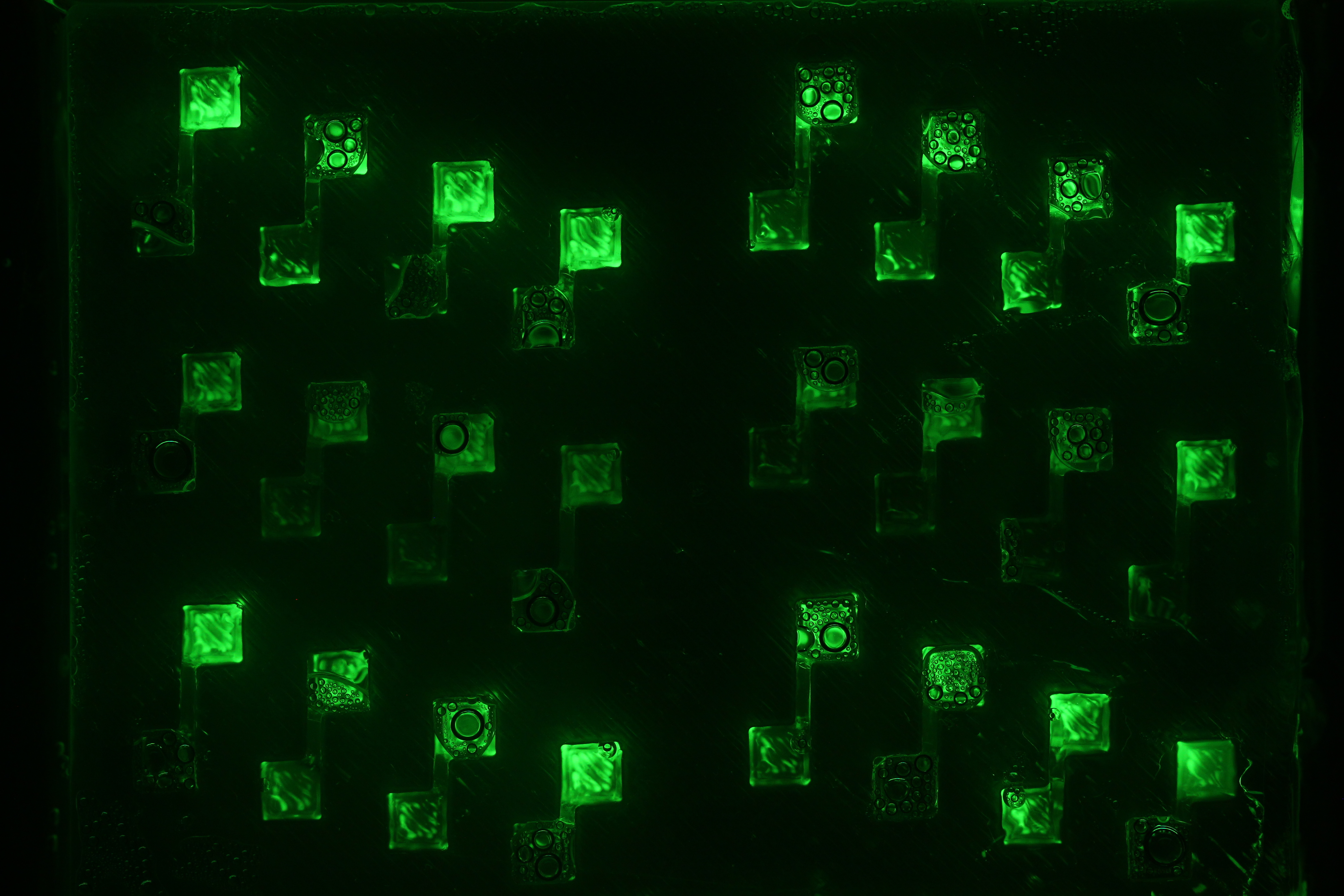
Diffusion experiment with liquid. Click here to watch the video.
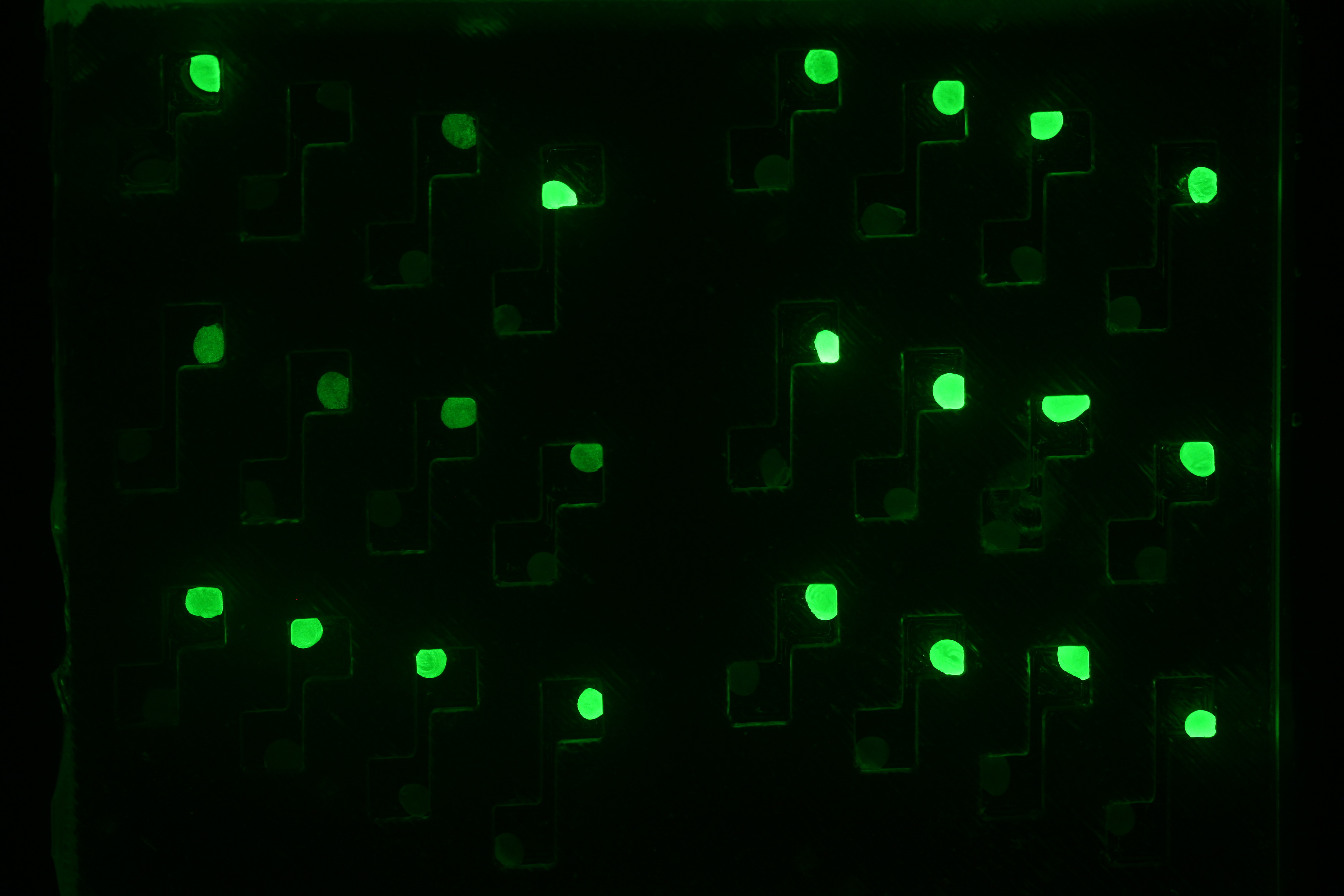
Diffusion experiment with beads. Click here to watch the video.
 "
"
