Team:Austin Texas/kit
From 2014.igem.org
Nathanshin (Talk | contribs) |
|||
| Line 83: | Line 83: | ||
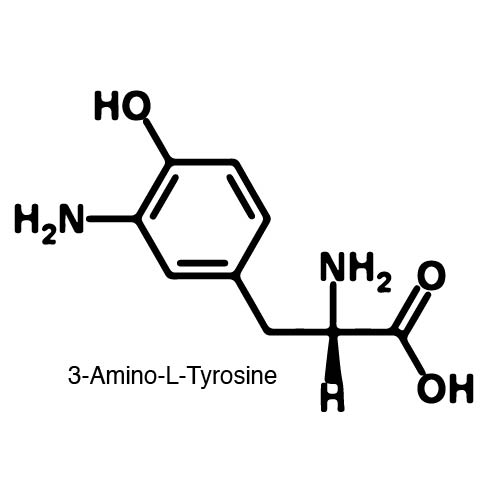
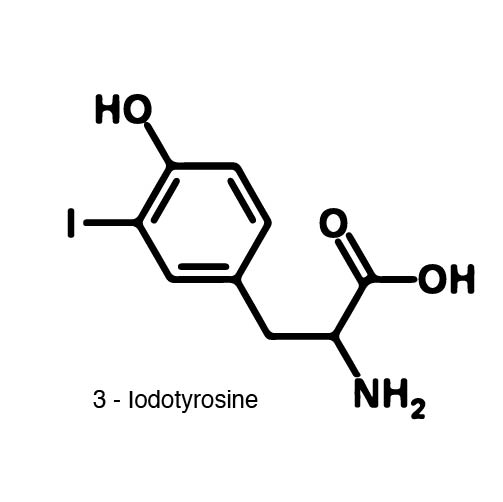
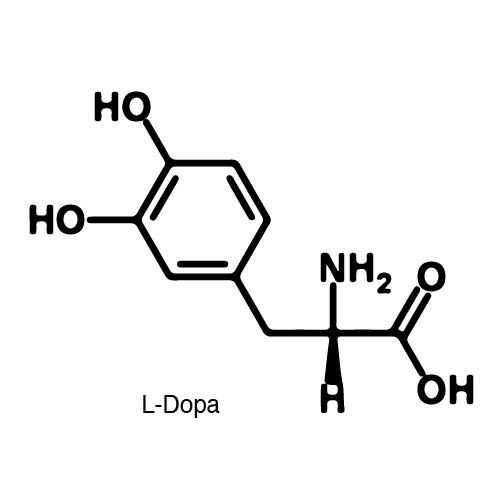
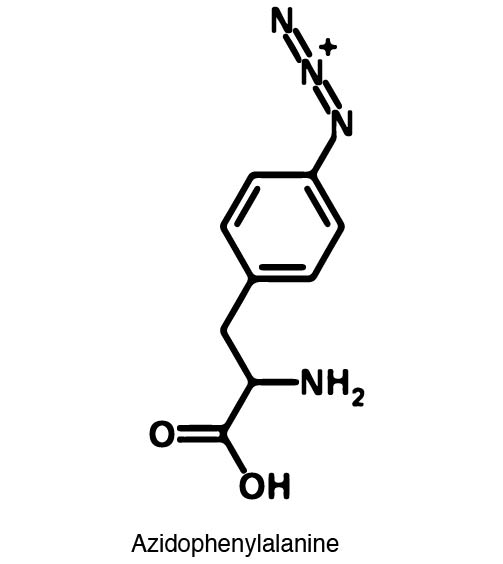
Complications arise when the genetic code is re-coded. Release Factor RF1 is responsible for terminating translation when the ribosome reaches the Amber stop codon. To avoid termination at UAG, a strain of E. Coli was engineered to remove the RF1 gene. During translation, the ncAA synthetase charges an ncAA onto the tRNA. Rather than translation termination, the tRNA enters the ribosome for ncAA incorporation and translation continues. Another complication occurs in the RFO strain of E. Coli due to the presence of Amber codons on the F plasmid. The F plasmid contains essential genes to the cell. The functionality of the proteins encoded would be compromised in a genetically expanded cell. To prevent this lethality, a strain of E. Coli with the RF1 knockout was engineered to have alternative stop codons on the F plasmid known as Amberless E. Coli. | Complications arise when the genetic code is re-coded. Release Factor RF1 is responsible for terminating translation when the ribosome reaches the Amber stop codon. To avoid termination at UAG, a strain of E. Coli was engineered to remove the RF1 gene. During translation, the ncAA synthetase charges an ncAA onto the tRNA. Rather than translation termination, the tRNA enters the ribosome for ncAA incorporation and translation continues. Another complication occurs in the RFO strain of E. Coli due to the presence of Amber codons on the F plasmid. The F plasmid contains essential genes to the cell. The functionality of the proteins encoded would be compromised in a genetically expanded cell. To prevent this lethality, a strain of E. Coli with the RF1 knockout was engineered to have alternative stop codons on the F plasmid known as Amberless E. Coli. | ||
| - | <h1> | + | <h1>Experimental Design</h1> |
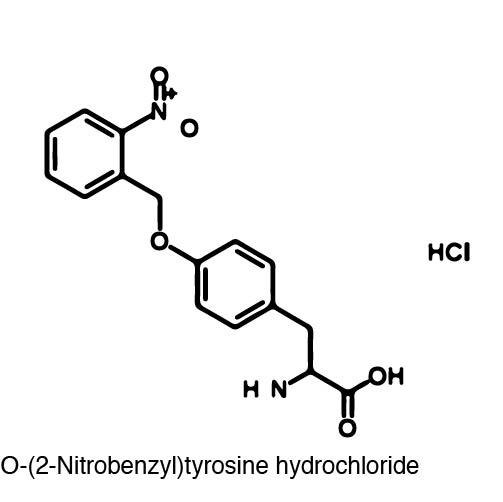
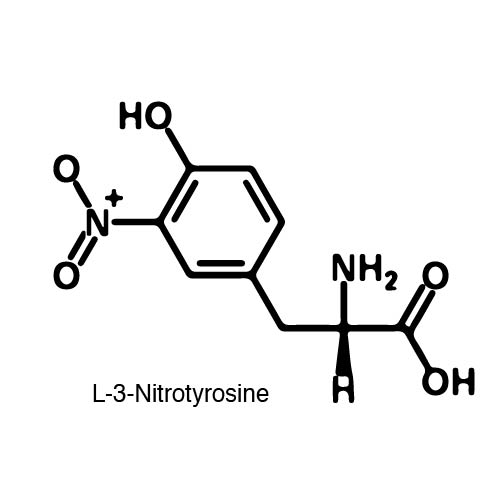
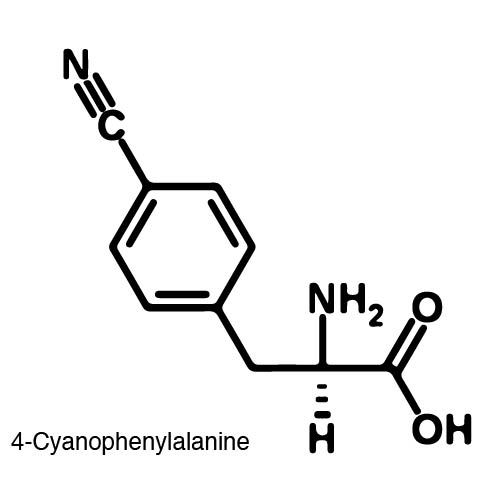
| - | In order to re-code UAG, a synthetase/tRNA pair much be modified to effectively grab onto an ncAA. Various methods of directed evolution are typically used to modify a synthase such that it can grab onto and charge a non-canonical. The library of ncAA synthetases available have a ranging levels of reported efficiency and are not well | + | In order to re-code UAG, a synthetase/tRNA pair much be modified to effectively grab onto an ncAA. Various methods of directed evolution are typically used to modify a synthase such that it can grab onto and charge a non-canonical. The library of ncAA synthetases available have a ranging levels of reported efficiency and are not well characterized. The This year the UT iGEM Team created a test kit designed to characterize the efficiency of an ncAA synthetase/tRNA system. |
| - | The | + | The kit consists of a three plasmid system: pBLG, pFRYC, and pFRY. pBLG contains a gentamicin resistance as well as the ncAA synthetase/tRNA pair. pFRYC is the control plasmid and contains a kanamycin resistance gene and two reporters connected via a linker sequence. pFRY is the experimental plasmid and is similar to pFRYC however the pFRY linker sequence contains an amber stop codon whereas the pFRYC linker does not. The two reporter genes are sfGFP (upstream the linker) and mCherry. In cell containing pFRYC, the ribosome will translate the sfGFP reporter, linker, and finally mCherry producing a fluorescent reporter of green and red resulting in a yellow fluorescent. In a cell containing pFRY, the ribosome will translate the sfGFP and terminate at the amber stop codon on the linker producing a green fluorescence. When pBLG and pFRY are present in the cell, the ribosome will incorporate an ncAA at the amber codon in the linker and continue translation producing both sfGFP and mCherry reporters if the synthetase/tRNA pair encoded on pBLG effectively incorporate the ncAA. |
Revision as of 20:49, 10 October 2014
|
 "
"