Team:Saarland/2 step
From 2014.igem.org
Step 2: Find allies and join forces

Skip to a chapter by clicking the corresponding marking above
Bacillus megaterium

B. megaterium belongs to the group of gram positive, aerobic bacteria. It’s typically found in soil, but can also survive in sea water or dried foods (Vary et al., 2007). Since its discovery in 1884, it has become one of the most important organisms for industrial and research purposes, because it combines numerous useful characteristics. Due to its extraordinary size of up to 1,5 µm x 4 µm and it`s impressive cell volume of more than 60 µm3, B. megaterium is predestined for studies regarding cell structure and protein localisation (Boyke et al., 2010; Vary et al., 1992). Furthermore it is able to metabolise more than 62 carbon sources, making its cultivation efficient and inexpensive (Millet et al., 1962). Probably the most useful characteristic for our purposes is its ability of preserving plasmids over multiple generations, making it a good specimen for the secretion of proteins and other organic molecules. Especially concerning the B. megaterium strain MS941 that was derived from the strain DSM319 by knockout of the neutral protease gene nprM (Wittchen et al. 1995). For this reason the non sporulating strain MS941 is ideal for heterologous protein expression. This was shown by overexpression of GFP (Stammen et al., 2007).
Additionally, the biosynthetic pathway of hyaluronic acid precursor molecules is already established in B. megaterium since UDP-N-acetyl-D-glucosamine and UDP-D-glucuronic acid are also essential components for cell wall synthesis in gram positive bacteria. Manual supplementation of these extremely expensive precursor molecules is not longer necessary. This could contribute to a future profitable biotechnological production of the naked mole rat's high molecular mass hyaluronic acid (HMM-HA). Homology searches in the B. megaterium genome have also shown that there are no endogenous hyaluronidases, which would otherwise immediately degrade the produced HMM-HA. Furthermore B. megaterium does not possess an endogenous hyaluronan synthase (Has). For this reason our team can be sure that hyaluronic acid has exclusively been synthesised by the correct enzyme and features the correct molecular weight and anti carcinogenic properties.
Pathway engineering
For the optimisation of hyaluronic acid production our team intends to overexpress endogenous B. megaterium proteins that are necessary for the production of hyaluronic acid precursor molecules. We think that the flow equilibrium of metabolites will be shifted towards production of HMM-HA, rather than to cell wall components. This approach could make a considerable contribution for high yield production of HMM-HA. The identification of corresponding genes in B. megaterium is based on the biosynthetic pathway for the HA production in group A and group C streptococci as well as for HA production in B. subtilis (Widner et al. 2005). Figure 2 shows the proposed biosynthetic pathway for production of the HA precursor molecules in B. megaterium according to in silico homology search on MegaBac v9 database.
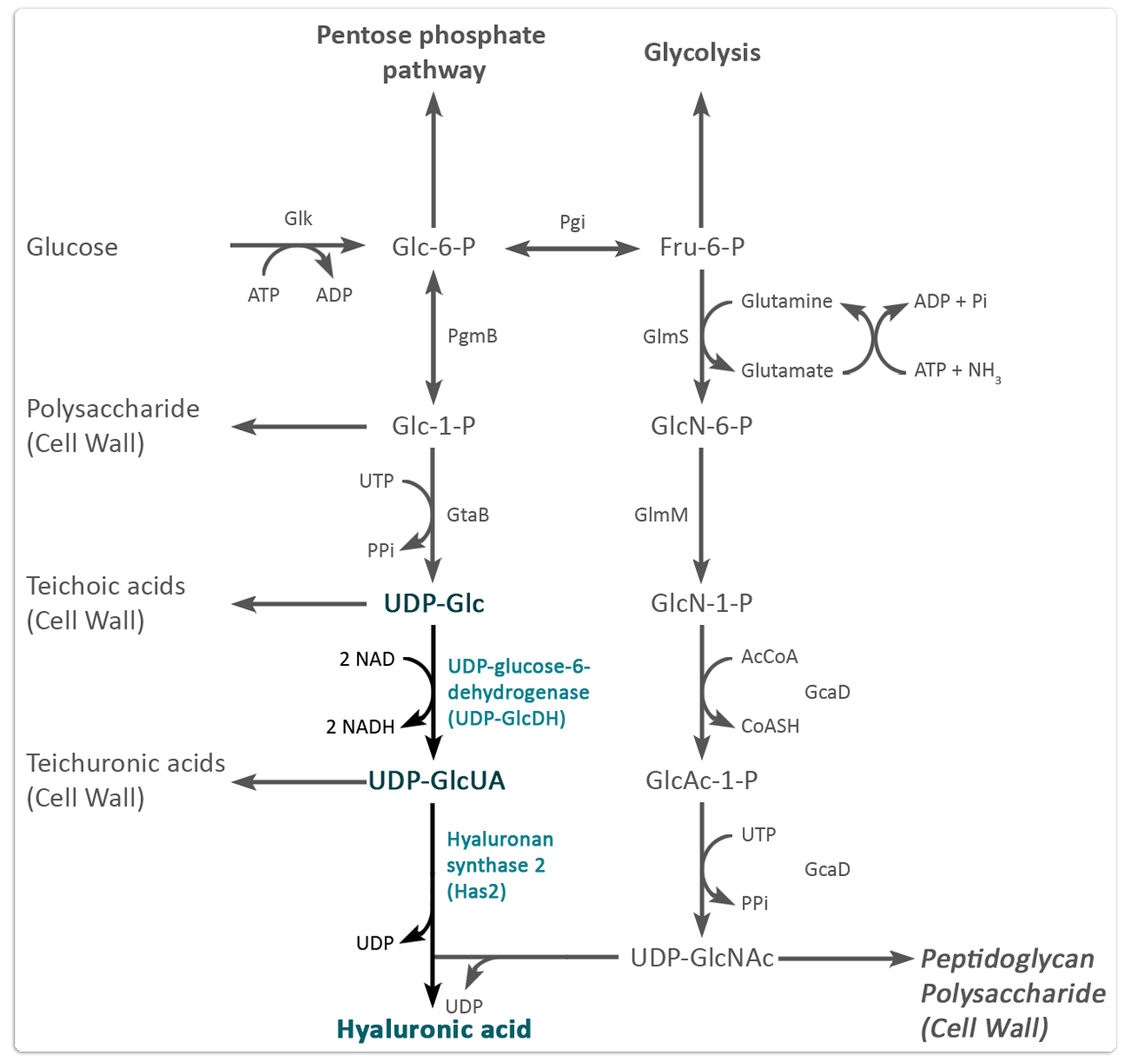
However, when the HA will be successfully produced as we described in figure 2, it is supposed to be secreted into the medium. Since studies with the compareable B. subtilis expressing a streptococci hyaluronan synthase (HasA) have already shown similar results. It’s either possible that there is a specific transporter for oligosaccharides mediating HA secretion into the medium, or that the multi transmembrane domains of the hyaluronan synthase themselves form a channel for the secretion of the nascent HA chain (Weigel, 2002).
References
Bunk, B., Schulz, A., Stammen, S., Munch, R., Warren, M.J., Rohde, M., Jahn, D., and Biedendieck, R. (2010). A short story about a big magic bug. Bioeng. Bugs 1, 85–91.
Schaeffer, P., Millet, J., and Aubert, J.P. (1965). Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. U. S. A. 54, 704–711.
Stammen, S., Müller, B.K., Korneli, C., Biedendieck, R., Gamer, M., Franco-Lara, E., and Jahn, D. (2010). High-Yield Intra- and Extracellular Protein Production Using Bacillus megaterium. Appl. Environ. Microbiol. 76, 4037–4046.
Vary, P. (1992). Development of genetic engineering in Bacillus megaterium. Biotechnol. Read. Mass 22, 251–310.
Vary, P.S., Biedendieck, R., Fuerch, T., Meinhardt, F., Rohde, M., Deckwer, W.-D., and Jahn, D. (2007). Bacillus megaterium—from simple soil bacterium to industrial protein production host. Appl. Microbiol. Biotechnol. 76, 957–967.
Weigel, P.H. (2002). Functional Characteristics and Catalytic Mechanisms of the Bacterial Hyaluronan Synthases. IUBMB Life 54, 201–211.
Widner, B., Behr, R., Dollen, S.V., Tang, M., Heu, T., Sloma, A., Sternberg, D., DeAngelis, P.L., Weigel, P.H., and Brown, S. (2005). Hyaluronic Acid Production in Bacillus subtilis. Appl. Environ. Microbiol. 71, 3747–3752.
Wittchen, K.D., and Meinhardt, F. (1995). Inactivation of the major extracellular protease from Bacillus megaterium DSM319 by gene replacement. Appl. Microbiol. Biotechnol. 42, 871–877.
 "
"



 Modelling
Modelling
 Previous Step
Previous Step

Impressum/Copyright