Team:Paris Saclay/Notebook/July/28
From 2014.igem.org
Contents |
Monday 28th July
Lab work
The frame coli Odor free
Preparation of electrocompetent cells
by Arnaud & Romain
Strain used: E. coli MG1655Z1 and E. coli MG1655.
Protocol:
Two dilution of 200µl of bacterial culture MG1655Z1 and E. coli MG1655 in 15ml of LB at 30°C for each strain.
When the culture OD650 = 0,6:
- put in ice during 10min.
- centriguge at 4°C, 5min, 4000rpm.
- Discard the supernatant, and resuspend the pellet in 15ml glycerol 10% COLD.
- centriguge at 4°C, 5min, 4000rpm.
- Discard the supernatant, and resuspend the pellet in 15ml glycerol 10% COLD.
- centriguge at 4°C, 5min, 4000rpm.
- Discard the supernatant, and resuspend the pellet in 200µl glycerol 10% COLD.
Transformation of electrocompetent cells
by Arnaud & Romain
Strain used: E. coli MG1655Z1 and E. coli MG1655. Plasmid used: BT340 (code for a flipase for E. coli)
Make 2 électroporations in cold electroporation cuvettes:
- A control cuvette(without DNA): 50µl of E. coli MG1655Z1 or E. coli MG1655.
- A second cuvette: 50µl of E. coli MG1655Z1 or E. coli MG1655 culture + 1µl of BT340 plasmid.
Electroporation : 2500V, 132W, 40µF.
After that, add 1ml of cold LB in each cuvette and transfer in 2 tubes.
Spread on 8 dishes LB + Cm:
- 20µl of control E. coli MG1655Z1 (without plasmid)
- 50µl of transformed E. coli MG1655Z1 with BT340.
- 100µl of transformed E. coli MG1655Z1 with BT340.
- Transformed and concentrated E. coli MG1655Z1 with BT340.
- 20µl of control E. coli MG1655 (without plasmid)
- 50µl of transformed E. coli MG1655 with BT340.
- 100µl of transformed E. coli MG1655 with BT340.
- Transformed and concentrated E. coli MG1655Z1 with BT340.
Incubate for a night at 30°C.
PCR
by Sean
Cultures used: MG1655Z1 10I and MG1655 100I.
Note: although we have 2 cultures to prepare, it is recommended to prepare 3 tubes because of probable uncertainties when measuring volumes.
Salicylate Inducible Suppressing System
Plasmid DNA Purification
by Fabio
- BBa_J61051 Cl.1
- BBa_J61051 Cl.2
- BBa_K228001 Cl.1
- BBa_K228001 Cl.2
from Bacterial Culture made the 25th July.
At the end of the process, we collected 4ml of each clone 1 plus 1ml of glycerol (80%) and stored at -80°C.
Digestion to check
by Fabio
Once we have a good amount of our BioBricks' plasmid, we did the first digestion (verification). Both BioBricks were digested with the enzyme NotI, that separates both BioBricks' core from theirs vectors. The graphic result of this process is shown in the next step.
Protocol:
- Add 1μl of the plasmid to digest.
- Add 2μl of buffer (Fast Digest Buffer 10x).
- Add 0,5μl of enzyme (in this case, NotI).
- Complete with 16,5μl of H2O.
- Mix gently.
- Incubate at 37°C for one hour.
Electrophoresis
by Fabio
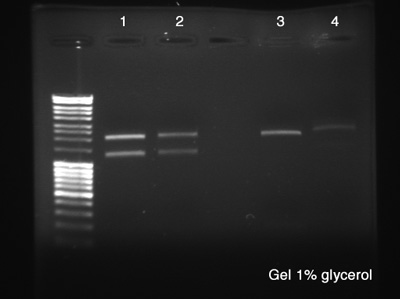
The Electrophoresis was used to verify the success of the Digestion and the success of the plasmid DNA purification at the same time.
- BBa_J61051 Cl.1
- BBa_J61051 Cl.2
- BBa_K228001 Cl.1
- BBa_K228001 Cl.2
Results:
- Success, we can clearly see both parts of the digestion product.
- Same conclusion of number 1.
- We can see one part of the digestion very clear and the other one (lower down) is almost invisible. We'll use a strong concentration in the future.
- Both parts are not strong. We'll discontinue this clone.
BioBrick Assembly - Segregate Process Protocol
Lemon scent
Liquid Culture
by Fabio
Due to an widespread infection of the cells made the 25th July, we collected the original strain DY330 to verify its legitimacy.
2ml LB + 20μl of DY330. We incubate cultures at 30°C.
Members present:
- Instructors and advisors: Solenne and Sylvie.
- Students: Arnaud, Fabio, Romain, Sean and Terry.
 "
"