Team:Paris Saclay/Notebook/July/21
From 2014.igem.org
(→3 - Oligonucleotide's design) |
(→Monday 21st July) |
||
| Line 3: | Line 3: | ||
==Lab Work== | ==Lab Work== | ||
| - | === | + | ====Results: Transformation of CaCl<sub>2</sub> supercompetent cells==== |
''by Romain'' | ''by Romain'' | ||
| Line 11: | Line 11: | ||
Results: Nothing has grown. | Results: Nothing has grown. | ||
| - | === | + | ====Results: Transformation of DY330 via pJBEI6409==== |
''by Sean'' | ''by Sean'' | ||
| Line 42: | Line 42: | ||
'''Conclusion: The increase in colony number is proportional to the increase in volume used. The control yielded nothing. The results are coherent.''' | '''Conclusion: The increase in colony number is proportional to the increase in volume used. The control yielded nothing. The results are coherent.''' | ||
| - | == | + | ===D - Banana scent=== |
| - | === | + | ====Oligonucleotide's design==== |
''by Romain'' | ''by Romain'' | ||
For the banana scent, design of iPS91 and iPS92, the ilvE sequence to transformed the Leucin into α-cetoisocaproate (The first step). | For the banana scent, design of iPS91 and iPS92, the ilvE sequence to transformed the Leucin into α-cetoisocaproate (The first step). | ||
| - | === | + | ====Liquid Bacterial Culture==== |
''by Marie, Romain & Sean'' | ''by Marie, Romain & Sean'' | ||
| Line 56: | Line 56: | ||
*The new strains received | *The new strains received | ||
| - | === | + | ====Electrophoresis==== |
''by Fabio (process A) and Mathieu (process B and C)'' | ''by Fabio (process A) and Mathieu (process B and C)'' | ||
Revision as of 14:13, 24 July 2014
Contents |
Monday 21st July
Lab Work
Results: Transformation of CaCl2 supercompetent cells
by Romain
Strains transformed: MG1655, MG1655Z1. from Bacterial Cultures transformed the 18th July
Results: Nothing has grown.
Results: Transformation of DY330 via pJBEI6409
by Sean
Transformation performed on the 18th July
| Volume of plasmid used | 50μl from cuvette | 100μl | remainder (850µl) |
|---|---|---|---|
| 2μl of plasmid | 0 | 4 | 30 |
| 4μl | 3 | 6 | 45 |
| Control | 0 | 0 | 0 |
Conclusion: The increase in colony number is proportional to the increase in volume used. The control yielded nothing. The results are coherent.
D - Banana scent
Oligonucleotide's design
by Romain
For the banana scent, design of iPS91 and iPS92, the ilvE sequence to transformed the Leucin into α-cetoisocaproate (The first step).
Liquid Bacterial Culture
by Marie, Romain & Sean
- DY330 pJBEI6409 with 10µl Cm in 10ml LB (x2)
- BT340 Cm and Amp
- The new strains received
Electrophoresis
by Fabio (process A) and Mathieu (process B and C)
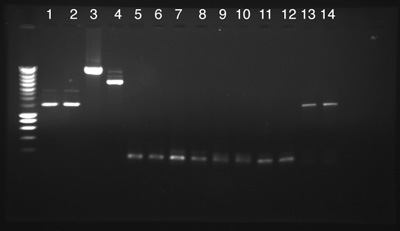
We used 2µl of DNA of the following Biobricks in a 1% Agarose Gel. The PCRs came from Mathias' manipulation made the 18th July.
Process A
- BBa_J23119 Cl1
- BBa_J23119 Cl2
- BBa_J23106 Cl1
- BBa_J23100 Cl2
- PCR 1
- PCR 2
- PCR 3
- PCR 4
- PCR 5
- PCR 6
- PCR 7
- PCR 8
- PCR 9
- PCR 10
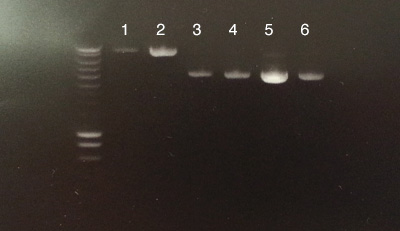
Process B
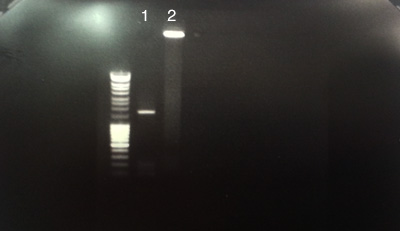
Process C
Pooling and purifying PCR 9 and 10 from process A.
- PCR purified products. IPS70 / IPS71 of pOsV230 (Apramycin)
- BT 340 (plasmid's flipase)
Results:
- A: From 1 to 4: Success, DNAs have the expected size.
- A: From 5 to 12: Failure, No PCR products.
- A: Numbers 13 and 14: Success, PCR products have the expected size.
- B: All 6 extractions were successful.
- C: Number 1: successful concentration of pOsV230's PCR product.
- C: Number 2 had no migration.
People there:
- Instructors and advisors: Solenne and Sylvie.
- Students: Arnaud, Fabio, Juliette, Mathieu, Marie, Pierre, Romain, Sean and Terry.
 "
"