Introduction
This part aims to simulate the circuit of Attachment part, which can help shorten the distance of helper E. coli and S. mutans.
The circuit is constructed by the following parts, constitutive promoter + rbs + INPNC (k523013) + C16 + terminator (B0015). The detailed mechanism is introduced on Cleanse-Attachment.

In this part, we fit the parameter in the system with the experimental data from our wet lab. To test how the circuit works, RFP is added after C16 in the testing circuit. It can represent the amount of C16 protein. The amount of protein expression is transferred from the RFP florescence [1].
System
INPNC, C16, and RFP are the main product in the testing circuit. In the design circuit, there are only INPNC and C16. RFP can be used to test whether the circuit is functional.
$$\frac{d[\text{RFP mRNA}]}{dt}=P_{const}-K_{deg_mRFP}[\text{RFP mRNA}]$$
$$\frac{d[\text{RFP}]}{dt}=K_t[\text{RFP mRNA}]-K_{deg_RFP}[RFP]$$
1. $P_{const}$:constitutive promoter activity
2. $K_{deg\_mRFP}$:RFP mRNA degradation
3. $K_{t}$:Translation efficiency
4. $K_{deg\_RFP}$:RFP degredation
Result
While fitting the parameter, we set range of parameter which met the range of normal promoter activity, mRNA degradation, translation rate, and protein degradation [2]. The result of fitted parameter is, $P_{const}=0.24$, $K_{deg\_mRFP}=0.15$, $K_{t}=19.45$, $K_{deg\_RFP}=0.01$. The result of simulation and experimental data is very similar.
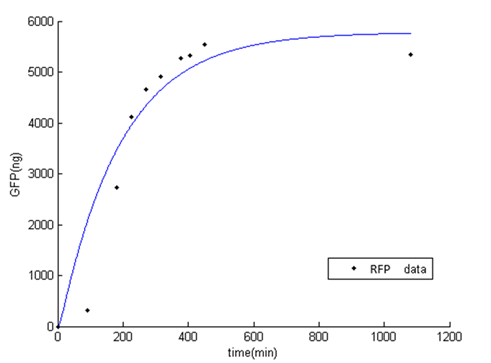
Figure 1: Result of RFP simulation and experimental data
Reference
- H. A. Richards, Quantitative RFP fluorescence as an indicator of recombinant protein synthesis in transgenic plants. Plant Cell Rep (2003) 22:117–121
 "
"