Team:NCTU Formosa/project
From 2014.igem.org
Contents
|
Motivation
Serious Problem in The World (Insect Damage)
From ancient times to the present, harmful insects always play roles in our life, and we don’t have any appropriate solution to these annoying pests. Every year, harmful insects cause a great amount of loss to our agriculture, which seriously disturbs people's livelihood and economy.
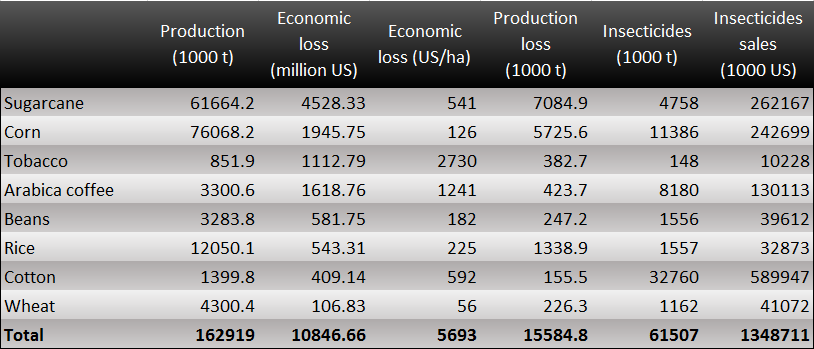
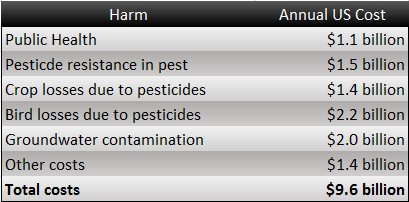
Take Brazil for example. We can find several crucial economic crops such as coconut, coffee, and sugarcane which are damaged by harmful insects. The total loss of crops in Brazil is about 11 billion US dollars. In addition, we can also find that people spent nearly 1.4 billion controlling these annoying pests. What's more? They have used a great number of insecticides containing some toxic substances. However, this method will make human beings exposed to horrible situations.
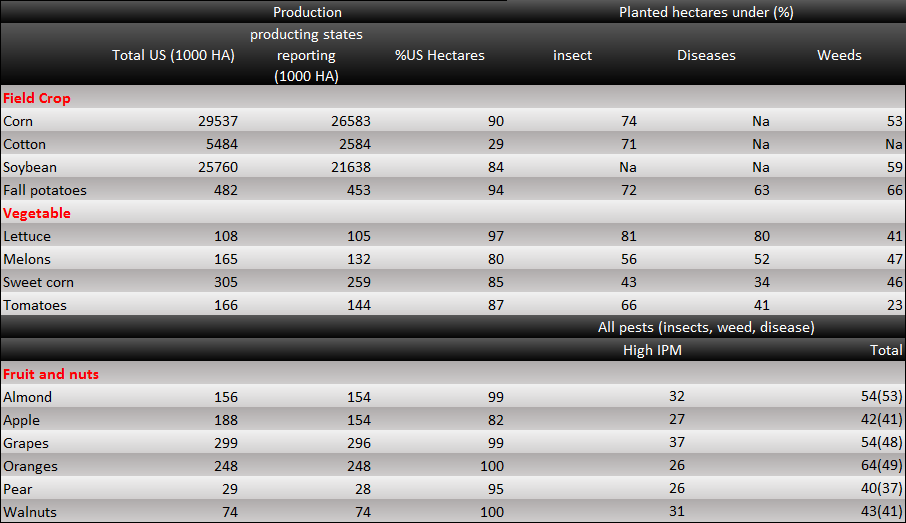
Also, there is a data about the IPM (Integrated pest management) of USA. IPM means the ability to control pests. The IPM is reality number in global, and the lower the number is, the more efficient we are able to control. In the data, we can see that crops are planted in lots of areas in USA. There are several kinds of plants which are still under attack by harmful insects. We can only control the insect damage efficiently for a small, limited area. Therefore, as the production areas expand to an extent, the effect of control declines exponentially. Even though the production areas become larger, the total production might be lower.
Therefore, what we need is a brand new method to solve this problem.
Common Methods of Solving Insect Damage Problem
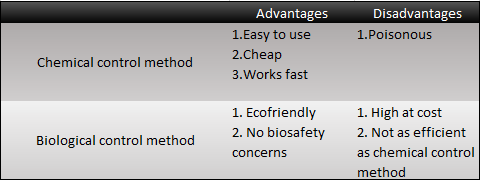
Chemical Control Method
Because of the hazard of insects, human beings have come up with lots of idea to kill these harmful insects. In the 15th century, people used heavy metals such as Arsenic, Mercury and Plumbum to kill harmful insects, which was a catastrophe to the environment. Pesticides became more powerful along with the technology. In the 20th century, the agriculture developed rapidly just because of the evolution of pesticides. But the pesticides are not only fatal to the insects but also harmful to the human beings. People found this problem after several decades. The toxin of the pesticides will be kept in creatures by the food chain, and eventually, enters human's body.
Damage to Human Body
Pesticides includes substances that kill weeds (herbicides), insects (insecticides), fungus (fungicides), rodents (rodenticides), and others. The use of toxic pesticides to manage pest problems has become a common practice around the world. Pesticides are used almost everywhere, causing human health hazards, ranging from short-term impacts such as headaches and nausea to chronic impacts like cancer and reproductive harm. There is also mounting evidence that exposure to pesticides disrupts the endocrine system, wreaking havoc with the complex regulation of hormones, the reproductive system, and embryonic development.
Damage to The Environment
Pesticides wreak havoc on the environment, threatening biodiversity and weakening the natural systems upon which human survival depends on. Pesticides have led to abnormal massive mortality of America's honeybees. The populations of bees have dropped by 29% to 36% each year since 2006. To our astonishment, approximately 1/3 of the food we eat depends on bees for pollination!
Secondly, some scientists believe that amphibians and bats have become more susceptible to deadly disease because their immune systems are weakened by pesticides. What's more, one kind of pesticides called herbicide, which can give a male frog a sex change. Genetically, the frogs are still males, but morphologically they are completely female and they can even mate successfully with other males and lay viable eggs. Pesticides have also contaminated waterways and endangered fish and birds, causing ecological damage.
As a matter of fact, pesticide resistance makes insecticides ineffective. Other factors in the speed with which a species evolves resistance are generation time and fecundity, that is, causing shorter generations and more offspring lead to resistance more quickly.
Biological Control Method
Since most chemical control methods are poisonous to the environment, humans began to look for alternative ways. One of the most well known example is bacillus thuringiensis (Bt), which can efficiently kill insects with its crystal proteins. However, recent researches have shown that more and more insects have been resistant to this kind of protein.
Chemical control method have caused fetal environmental damage and insects have grown resistant to both the chemicals substances and Bt. It is, however, not too late to improve this situation. We can create an agricultural evolution with our new method!
Pheromone trap is currently the most novel method to solve the insect damage problem. What's more, pheromone is a pollution-free substance that is produced by the insects, meaning that insects cannot grow resistant to it. This is why we initially took biological synthesis of pheromone in E.coli into consideration.
Novel and Efficient Ways to Solve Insect Damage Problem -
The Pheromone Trap
Introduction of Pheromones
A pheromone is a secreted or excreted chemical factor that triggers a social response among members of the same species. Pheromones are capable of acting outside the body of the secreting individual to impact the behavior of the receiving individuals.
There are many kinds of pheromones such as alarm pheromones, food trail pheromones, sex pheromones, and many others that affect behavior or physiology.
Sex pheromones are an important factor in finding a mating partner. When a female releases chemicals, the mating search is initiated, and the male moths begin their upwind motion toward their potential partner. Pheromones that have the ability to propel long-distances and are emitted by the females abdominal glands in most cases.
How to Make Pheromones?
There are 2 main ways to make pheromones. One through chemical synthesis, the other one through biosynthesis approach.
Biosynthesis Pathway in Insects
Biosynthesis approach requires the cooperation of different enzymes, which is too complicated for E.coli to carry out. In addition, E.coli lacks Glycoproteins that can do posttranslational modification. Therefore, it is nearly impossible for E.coli to produce pheromones biosynthetically.
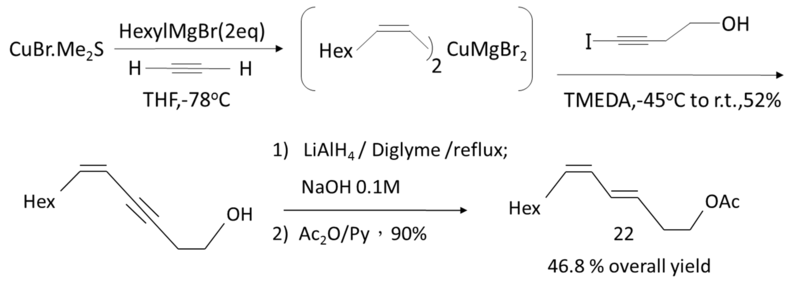
Chemical Synthesis Approach Used in Factory to Mass Produce Pheromone
It is difficult to make pheromone by chemical synthesis approach. This approach needs to use many kinds of chemical compound which may cause some pollution to the environment. Furthermore, the control of reacting condition may consume a lot of energy. An example of chemical synthesis approach: leafroller moth, Bonagota cranaodes.
Difficulty and Disadvantage of Pheromone Production
As mentioned above, pheromone is too complicated to be produced either artificially or bio-synthetically. Although the cost of artificial chemically synthesis can be lowered through massive production, the environmental damage it causes is fatal. With that said, we decided to find an easier and better way to reach our goal.
Idealist Method of Solving Insect Damage Problem - PBAN
To solve the impossibility of biosynthesizing pheromone with E.coli, we still managed to find another feasible way. As mentioned above, our method should be pollution-free, efficient, insect resistance-free, and cheap. Combining all these advantageous elements, we introduce you PBAN. Click the photos to learn more!
-
Cabbage MothMamestra brassicae
-
Cotton bollwormHelicoverpa armigera (Hubner)
-
Oriental Leafworm MothSpodoptera litura
-
Red imported fire antSolenopsis invicta
-
Yellow fever mosquitoAedes (Stegomyia) aegypyi
-
Black cutwormAgrotis ipsilon
-
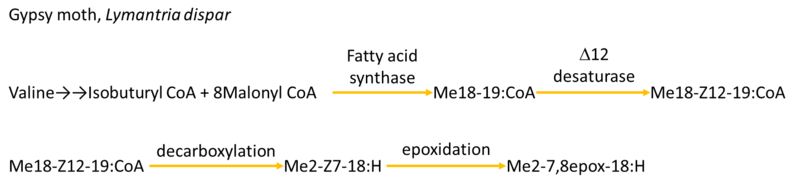
Gypsy MothLymantria dispar
-
LeafrollersStatherotis leucaspis Meyrick
-
SilkwormBombyx mori
Spread:This moths has a natural range across Europe, Asia, and North Africa.
Characteristics:The larva is green, khaki, grey-brown or brown with dark spots(1)(2)(3). The topside is darker than the bottom side and a yellow or light brown stripe goes round the middle portion by the spots.
Damage:The caterpillar of this species is seen as a pest for commercial agriculture. Often referred to as the "imported cabbageworm" they are a serious pest to cabbage and other mustard family crops. It can also be a pest of cultivated brassicas and sweet peas, but it feeds on a wide range of other plants.
Control: Organic controls(4) for cabbage worms include handpicking, excluding them with row cover barriers, or treating with a Bt pesticide. Cabbage worms are found throughout North America, and more than one species may be found in the same garden.
Spread:The pink bollworm has spread to cotton-growing regions throughout the world.
Characteristics: The larva is green, khaki, grey-brown or brown with dark spots(5). The topside is darker than the bottom side and a yellow or light brown stripe goes round the middle portion by the spots(32).
Damage: The cotton bollworm is a highly polyphagous species.[6] The most important crop hosts are tomato, cotton, pigeon pea, chickpea, sorghum and cowpea. Other hosts include groundnut, okra, peas, field beans, soybeans, lucerne, Phaseolus spp., other Leguminosae, tobacco, potatoes, maize, flax, Dianthus, Rosa, Pelargonium, Chrysanthemum, Lavandula angustifolia, a number of fruit trees, forest trees and a range of vegetable crops(6).
Control: Cultural controls, with the exception of the use of Bt cotton and the use of mating disruption and sprays of the Entrust formulation of spinosad are acceptable to use on organically grown cotton(7).
Spread:Widely distributed in Asia and Oceania.
Asia: Afghanistan, Bangladesh, Cambodia, China, Hong Kong, Indonesia, India, Iran, Japan, Laos, Malaysia, Myanmar, Nepal, North Korea, Oman, Pakistan, Philippines, Singapore, South Korea, Sri Lanka, Taiwan, Thailand, Vietnam. Oceania: Australia, Guam, New Caledonia, New Zealand, Micronesia, Papua New Guinea,
Samoa, other Pacific islands. United States: Hawaii(8).Characteristics: Adult moths measure between 15-20 mm (0.59-0.79 inches) in length and have a wingspan of 30-38 mm (1.18-1.5 inches). Forewings are gray to reddish-brown, with a complex pattern of creamy streaks and paler lines along the veins. Hind wings are grayish-white with grayish-brown margins. Males have a blue-grey band from the upper corner (apex) to the inner margin of each forewing. Larvae have bright yellow stripes along the back and the sides. Larval color varies from pale green to dark green(9)(31).
Damage: Oriental Leafworm Moth Spodoptera litura is a Noctuid moth which is considered as an agricultural pest. It is also known as the Cluster caterpillar, Cotton leafworm, Tobacco cutworm, and Tropical armyworm. It has a very wide host range of over 120 plant species, including: lettuce, cabbage, beetroot, peanuts, geranium, cotton, banana, fuchsias, acacia, African oil palm, amaranth, alfalfa, strawberry, sorghum, sugarcane, tomatoes, asparagus, apple, eggplant, beet, beans, broccoli, elephants ear, horsetail she oak, corn, flax, lantana, papaya, orange, mango, leek, among many others.
Control: The use of Bacillus thuringiensis (BT) may effectively control this pest. Other forms of biological, horticultural, and cultural control that have been studied include: planting near derris and garlic plants, breeding resistant plants from wild plants for example groundnuts from wild groundnuts, breeding resistant plants using bacterium Bacillus thuringiensis genes, using a Baculovirus, using the nematode Steinernema carpocapsae, and using the fly Exorista japonica(10).
Spread:The red imported fire ant, a eusocial species, are far more aggressive than most ant species. Animals, including humans, often encounter them by inadvertently stepping on one of their mounds, which causes the ants to swarm up the legs, attacking en masse. The ants respond to pheromones released by the first ant that attacks, thereafter stinging in concert(12).
Characteristics: Fire ants are red and black in coloration and, like all insects, they are protected by a hard exoskeleton and have six legs. Worker ants have round heads with mandibles, an armored thorax midsection and an abdomen, made up of the pedicle and the gaster. The head is typically copper brown in color. In addition to their mandibles, fire ant workers also possess an abdominal stinger(11)(29).
Damage: They are considered to be a pest, not only because of the physical pain they can inflict, but also because their mound-building activity can damage plant roots, lead to loss of crops(12).
Control: Hot water Pouring hot water on the mounds is effective and environmentally friendly, but may require 3 or 4 applications to kill the colony. Water should be at least scalding hot, but does not need to be boiling. This works best when you use 3 to 4 gallons of water in each application. WARNING: Hot water kills grass and shrubbery and may cause severe burns if spilled. Liquid nitrogen: it could be the most effective and most environmental friendly method to eradicate the species(30)(13).
Spread:The yellow fever mosquito, Aedes aegypti, is a mosquito that can spread the dengue fever, chikungunya, and yellow fever viruses, and other diseases. The mosquito can be recognized by white markings on its legs and a marking in the form of a lyre on the thorax. The mosquito originated in Africa, but is now found in tropical and subtropical regions throughout the world(14).
Characteristics: The mosquito can be recognized by white markings on its legs and a marking in the form of a lyre on the thorax.
Damage: The yellow fever mosquito, Aedes aegypti, is a mosquito that can spread the dengue fever, chikungunya, and yellow fever viruses, and other diseases(15).
Control: Empty water from containers such as flower pots, birdbaths, pet water dishes, cans, gutters, tires and buckets regularly to disrupt the mosquito breeding cycle. Consider using an insect repellent, be sure to follow the label directions for applying the repellent. For help selecting a mosquito repellent, try our Insect Repellent Locator(16).
Spread: This Caterpillar can be found, as various species, through be serious foliage feeders on some crops such as peanuts.hout North America.
Characteristics: Cutworms common in Georgia fields are black (Agrotis ipsilon (Ashmed)), granulate (Agrotis subterranea (Fabricius)) and variegated cutworm (Peridroma saucia(Hubner)). These are moths in the family Noctuidae. Full-grown cutworm larvae are 1.5 to 2 inches long. Coloration will vary among species, but all tend to be stout-bodied caterpillars with four sets of prolegs. They have the tendency to curl into a ball when disturbed(17).
Damage: Almost any plant can be attacked in the seedling stage. Cotton and certain vegetables sometimes have stand reductions(17).
Control: Bacillus thuringiensis, a widely available caterpillar-killing bacterium,is a very effective control for climbing cutworms as well as for the surface feeders(18).
Spread: It has a range which covers Europe, Africa, and North America(19).
Characteristics:Gypsy moth caterpillars change appearance as they grow. Young caterpillars are black or brown and about ¼ inch (0.6 cm) in length. As they grow, bumps develop along their backs along with coarse, black hairs. Each of the 11 sections of a developed caterpillar will have two coloured spots, the first five pairs, blue, and the last six, red. Mature caterpillars can be as long as 2 ½ inches (6.35 cm)(20).
Damage: It is classified as a pest, and its larvae consume the leaves of over 500 species of trees, shrubs and plants. The gypsy moth is one of the most destructive pests of hardwood trees in the eastern United States.he gypsy moth was considered a nuisance just ten years after their release. It included an account of all the trees being defoliated, caterpillars covering houses and sidewalks and that the caterpillars would rain down upon residents. The first outbreak occurred in 1889. An eradication program was begun in 1890.
Control: Tanglefoot Pest Barrier or Sticky Tree Bands can be placed around tree trunks to help curtail the caterpillars movement into and out of the tree canopy. Apply Bacillus thuringiensis, var. kurstaki or Monterey Garden Insect Spray (Spinosad) to the leaves of trees to kill gypsy moth caterpillars(21).
Spread: The obliquebanded leafroller (OBLR) is native to and widely distributed throughout temperate North America(22).
Characteristics: hatched larvae have a yellowish green body and a black head and thoracic shield. Mature larvae are 20 to 25 mm in length and the head and thoracic shield may be either black or various shades of brown(23).
Damage: Leafrollers, the larvae of certain tortricid moths, often feed and pupate within the protection of rolled-up leaves. Several species can cause problems on fruit and ornamental trees in California. The fruittree leafroller, Archips argyrospila, is the most common leafroller pest in landscapes throughout the state. It occurs on many ornamental trees—including ash, birch, California buckeye, box elder, elm, locust, maple, poplar, rose, and willow—and is particularly damaging to deciduous and live oaks. It also attacks numerous fruit and nut trees including almond, apple, apricot, caneberries, cherry, citrus, pear, plum, prune, quince, and walnut(24).
Control: Several parasites attack OBLR larvae but do not adequately control the pest. Apply sprays during June to kill the first summer brood adults and newly hatching larvae(24).
Spread: the place where farmers want to feed.
Characteristics : It is entirely dependent on humans for its reproduction and does not occur naturally in the wild(26).
Damage: None
Control: Not necessary
Reference
- Cabbage Moth - Caterpillar". Habitas.org.uk. Retrieved 2014-08-11.
- Interesting (To Us) Photos From The Garden". Meades.org. Retrieved 2011-08-11.
- RXwildlife Sightings » Blog Archive » More Emergence". Rxwildlife.org.uk. 2009-06-03. Retrieved 2011-08-11.
- Practice Organic Cabbage Worm Control for a Chemical-Free Garden, by Barbara Pleasant, March 25, 2013
- 2003-2009 Project «Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Economic Plants and their Diseases, Pests and Weeds
- Report of a Pest Risk Analysis Helicoverpa armigera, Hübner, 1808
- UC IPM Pest Management Guidelines: Cotton, http://ucanr.edu/sites/ipm/pdf/pmg/pmgcotton.pdf
- T.E.R.R.A.I.N, Spodoptera litura (NZ Tropical Armyworm) , http://www.terrain.net.nz/friends-of-te-henui-group/moths/moth-nz-tropical-armyworm-spodoptera-litura.html
- Effect of tetra hydroxyl-ρ-benzoquinone on growth and metamorphosis of Spodoptera litura Fabr. (Lepidoptera: Noctuidae) larvae, Sujata Magdum , Seema Gupta
- Espinosa, A. and A.C. Hodges University of Florida, Spodoptera litura, BUGWOODWiki, http://wiki.bugwood.org/Spodoptera_litura.
- OROKIN, Fire Ant Anatomy, http://www.orkin.com/ants/fire-ant/fire-ant-anatomy
- Wikipedia, Red imported fire ant http://en.wikipedia.org/wiki/Red_imported_fire_ant
- Organic Gardening.com/learn-and-grow/fire-ant-control</li?
- Cram101 Textbook Reviews, Life: The Science of Biology 8th Edition
- Wikipedia, Aedes aegypti , http://en.wikipedia.org/wiki/Aedes_aegypti
- National Pesticide Information Center, Mosquito Control Methods
- BugwoodWiki , Cutworms, by Dr. Steve L. Brown, Dr. Will Hudson
- Eliminate Cutworms Using Natural Pest Control By Susan Glaese March/April 1987
- Wikipedia, Lymantria dispar dispar , http://en.wikipedia.org/wiki/Lymantria_dispar_dispar
- FACT SHEET : GYPSY MOTH, http://www.metro-forestry.com/wp-content/uploads/2010/02/FactSheet_Gypsy-Moth.pdf
- Planet Natural, GYPSY MOTH
- Larry P. Pedigo, G. David Buntin, Handbook of Sampling Methods for Arthropods in Agriculture
- L. A. Hull, D. G. Pfeiffer & D. J. Biddinger, Apple Direct Pests
- IPM, obliquebanded leafroller, http://www.nysipm.cornell.edu/factsheets/treefruit/pests/oblr/oblr.asp
- UC IPM Online, Leafrollers on Ornamental and Fruit Trees, Publication 7473 September 2010
- Tan Koon San, Dynastic China: An Elementary History
- Pogue, Michael. "A review of selected species of Lymantria Huber [1819]". Forest Health Technology Enterprise Team. Retrieved September 14, 2012.
- Laurence Mousson, Catherine Dauga, Thomas Garrigues, Francis Schaffner, Marie Vazeille & Anna-Bella Failloux (August 2005). "Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations". Genetics Research
- M. S. Ascunce et al., “Global Invasion History of the Fire Ant Solenopsis invicta”, Science, vol. 331, no. 6020, pp. 1066 - 1068, 2011
- Scientists suggest fighting fire ants with ice By Chiu Yu-Tzu / STAFF REPORTER
- Capinera, J.L. 1999. Fall armyworm Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae)
- Helicoverpa Diapause Induction and Emergence Tool Introduction to the Helicoverpa armigera Genome Project Helicoverpa armigera Genome Project updates on InsectaCentral Helicoverpa Genome Project database on-line Lepiforum Funet Taxonomy Fauna Europaea
- Authored by W. H. Reissig. Published by the New York State Agricultural Experiment Station, Geneva, A Division of the New York State College of Agriculture and Life Sciences, A Statutory College of the State University, Cornell University, Ithaca. Funded in part by an Extension Service-USDA, IPM Grant.
PBAN ( Pheromone Biosynthesis Activating Neuropeptide )
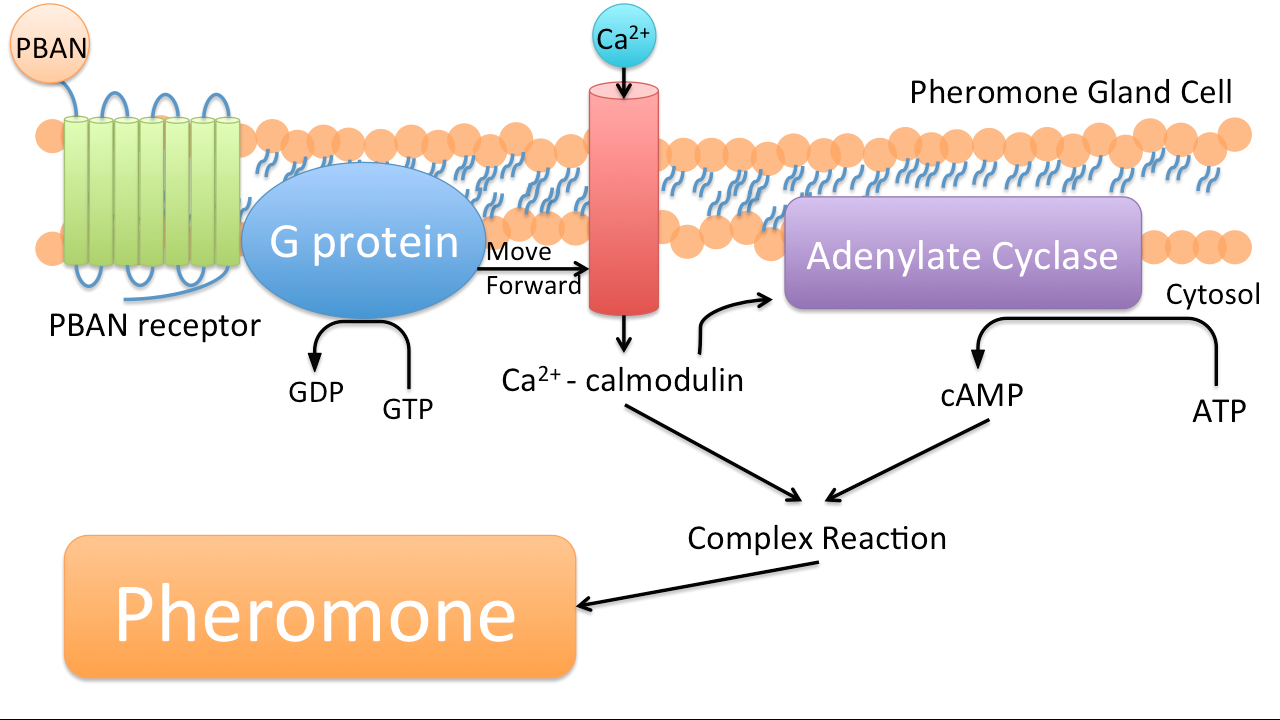
PBAN (Pheromone Biosynthesis Activating Neuropeptide) is a kind of peptide that can activate biosynthesis of pheromones of the targeted insects. Once a PBAN binds with the G-protein coupled receptor located at an insect’s pheromone gland, the signal sent by the G-protein coupled receptor activates kinase and phosphatase, which in turns activate other enzymes that participate in the biosynthesis of insect sex pheromones. These pheromones are eventually emitted.(34)
In nature, female insects such as moths release PBAN during mating to stimulate the synthesis of pheromones in order to attract male moths. PBAN can also facilitate the release of non-sex pheromones such as trail pheromones for ants.
Features of PBAN
1. Species-Specific: PBAN is species-specific just like pheromones, meaning that every kind of insect produces specific PBAN that only binds with it's targeted receptor, resulting in the production of a particular pheromone.
2. Small Simple Peptide: The coding sequence for a PBAN is only around 100 base pairs. To E.coli 100 base pairs is totally within its working capacity. and therefore, E.coli can be our low-cost PBAN factory. By synthesizing the DNA sequences for different PBAN into our factory, we can even produce a verity of PBANs. In addition, this factory is totally environmental friendly, unlike any pesticidewe have seen.
3. Insects' own secretion: Because PBAN is a insect's own secretion, insects could not form resistance it. In addition, it can easily trigger pheromone production by coming in contact with its receptor.
Our Main Idea
We plan to produce PBANs and make them come in contact with the target insect. The target insect will then start producing pheromones and attract more of the target insects for us.
PBAN Can Indeed Lead to Pheromone Production
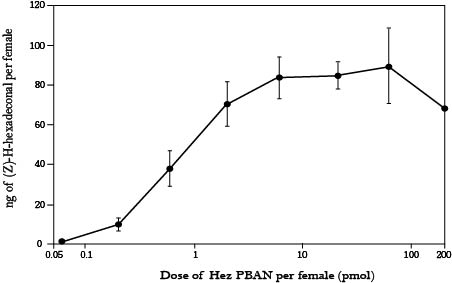
Before we could employ PBAN to accomplish our goals, we need to solve few problems. First, we had to confirm that whether an insect can take in vitro PBAN and allow it to function. From “Pheromonotropic Activity of Naturally Occurring Pyrokinin Insect Neuropeptides (FXPRLamide) in Helicoverpa zea” of ELSEVIER(36), this method has been proven possible. In this experiment, 0.05 pmol to 200 pmol of PBAN had been injected in to corn earworm and the amount of pheromone produced had been measured. The result (Fig.1-2-4) showed that PBAN can effectively increase the production of pheromone. The maximized amount of pheromone production was reached with only 2 pmol of PBAN. This experiment also showed that, as long as PBAN enters the worm’s body, it can function, whether it is injected or fed.
Problem of being degraded by peptidase
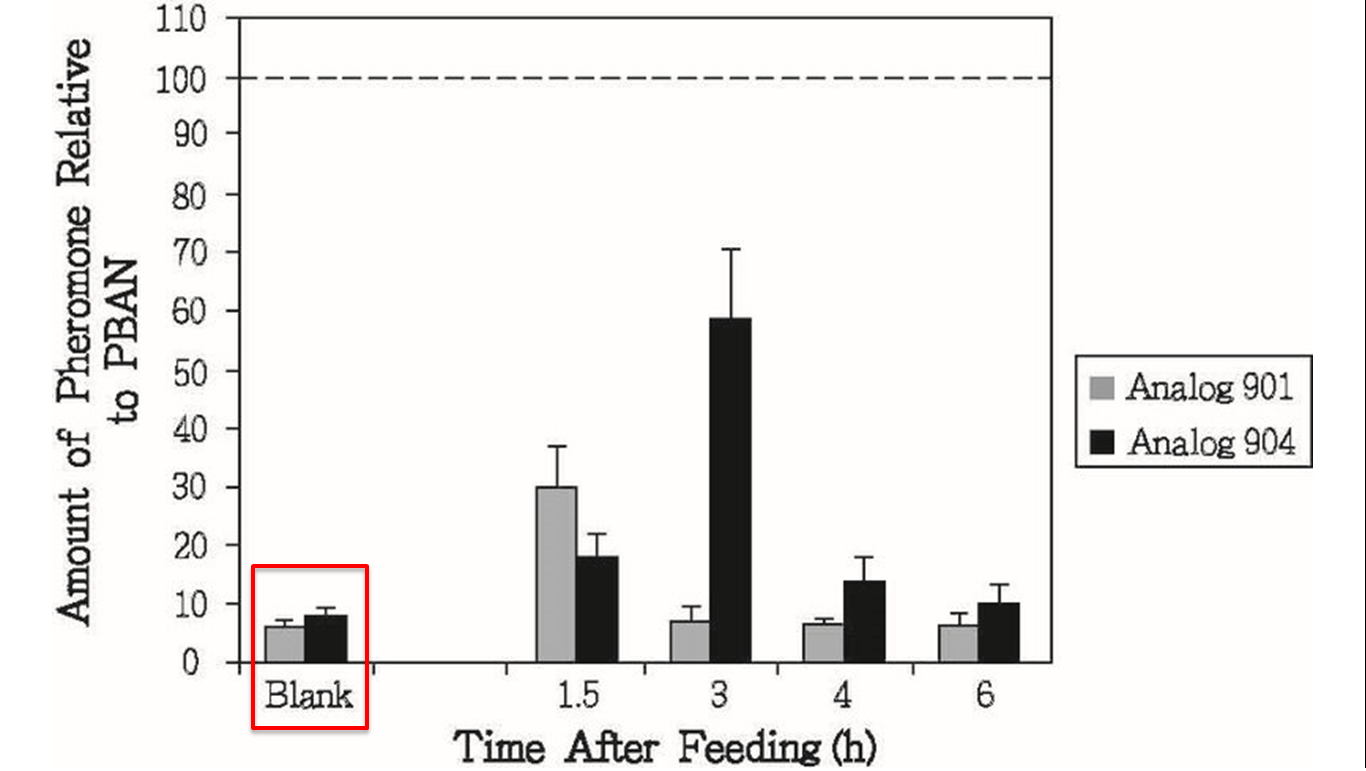
Our second problem is that PBAN would be degraded quickly in a insect’s body. In order to solve this problem, we search some former papers and we found another paper from ELSEVIER, “Enhanced oral availability/pheromonotropic activity of peptidase-resistanttopical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides.”(35) In this experiment, Heliothis virescens was used and amphiphilic analogs of pyrokinin/PBAN was used instead of natural PBAN. This way, the analog can resist peptidase, as shown in the graph below. The amount natural PBAN decreases quickly while the analogs degrades slowly (Fig.1-2-5).
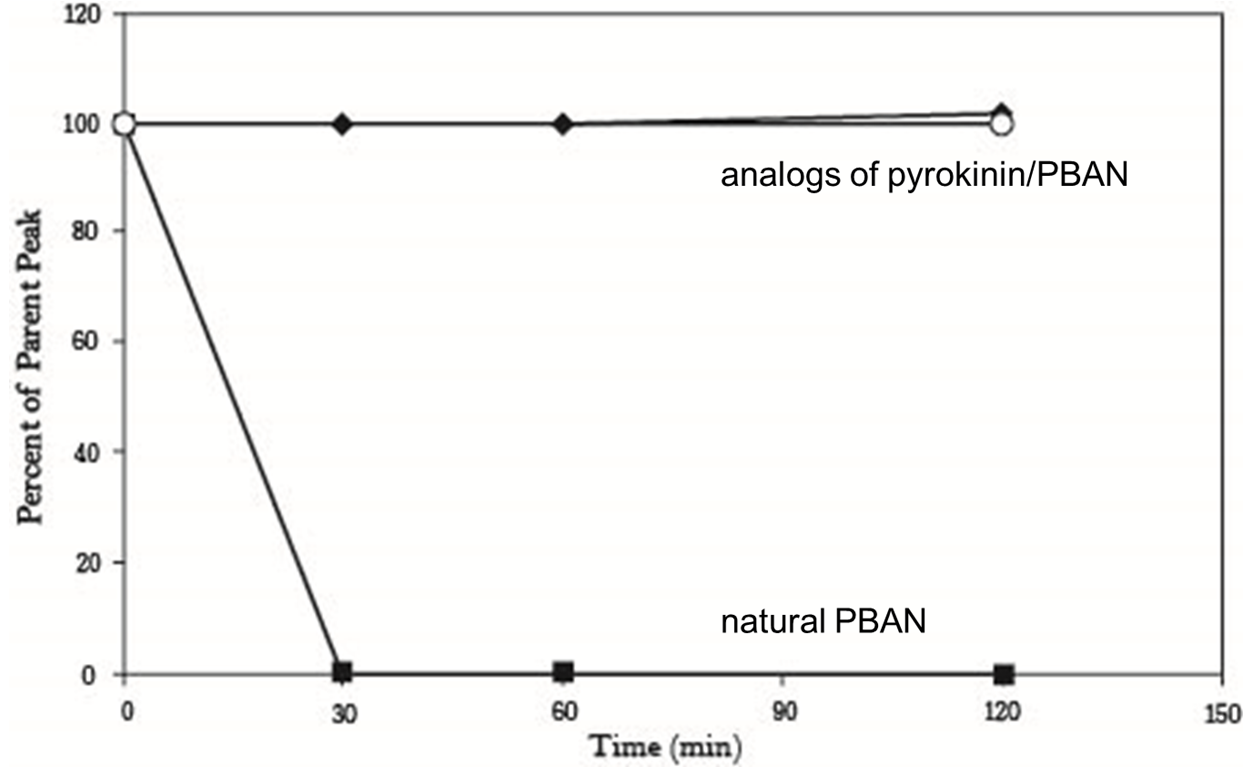
Solution ( Inspired by Paper, Led to Our Design )
This results above sounded not good for us, but there was other experiment interested us in the same research, researchers also conducted oral test with their PBAN (Fig.1-2-6), in this experiment, they fed moths with sugar solution which contained their PBAN analogs, and measure the amount of pheromone produced over time, that gave us inspiration, although natural PBAN can’t be maintained for a long time in moth’s body, we could solve this problem by feeding moths with PBAN continually, after combining this thought with the method of the oral experiment, we figured out an idea.
It is very possible that the PBAN in the food will penetrate into moths’ body from the digestive system. Thus, if female moths suck the food, PBAN in the moths’ body will be replenished, which solves the problem that PBAN can not maintain for a long time in the moths’body. As to how we produce PBAN and apply our concept to capture wanted harmful insects, we will explain in the following.
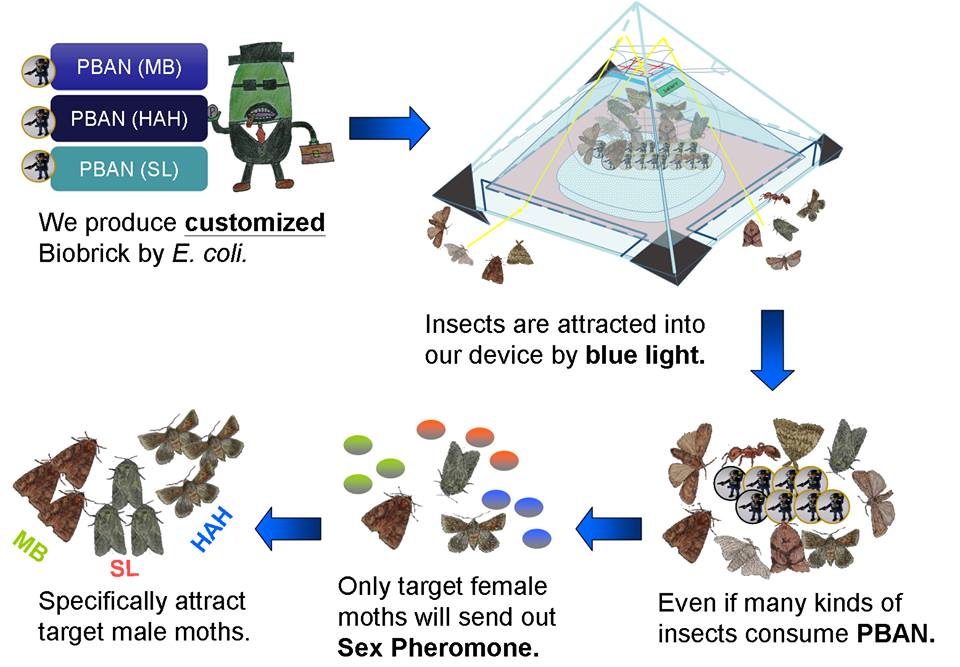
How We Are Going to Use PBAN?
In our project, we will biologically synthesize PBAN with our E.coli. We store the PBAN inside a trapping device (check this out at our Device page). In the device, there will be appropriate lighting and nutrient sources that will attract insects.
Once an insect is attracted into our device and ingests the nutrient sources we provide, it will also inevitably come in contact with our PBAN. As the PBAN works its magic and activates the pheromone synthesis of the attracted insect, more of this species of insect’s counterparts will be attracted and later captured.
Owing to the first feature mentioned above, PBAN are species-specific, which means that it doesn't matter if other kind of insect fly into our device and eat PBAN, because the insects we don't want to catch will not be stimulated by PBANs to produce pheromone; our PBAN are only for what we want to catch, and we are sure that our method won't affect other kinds of insects.
Reference
- Ada Rafaeli, Pheromone biosynthesis activating neuropeptide (PBAN): Regulatory role and mode of action, ELSEVIER, General and Comparative Endocrinology 162 (2009) 69–78.
- Ronald J. Nachmana, Peter E.A. Teal, Allison Strey, Enhanced oral availability/pheromonotropic activity of peptidase-resistant topical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides, ELSEVIER, Peptides 23 (2002) 2035–2043.
- RELLA L. ABERNATHY, RONALD J. , PETER E. A. TEAL, OKITSUGU YAMASHITAS AND JAMES H. TUMLINSON, Pheromonotropic Activity of Naturally Occurring Pyrokinin Insect Neuropeptides (FXPRLamide) in Helicoverpa zea, ELSEVIER, Peptides, Vol. 16, No. 2, pp. 215-219, 1995.
Biobrick Design
Basic Biobrick Design to Use Our PBA
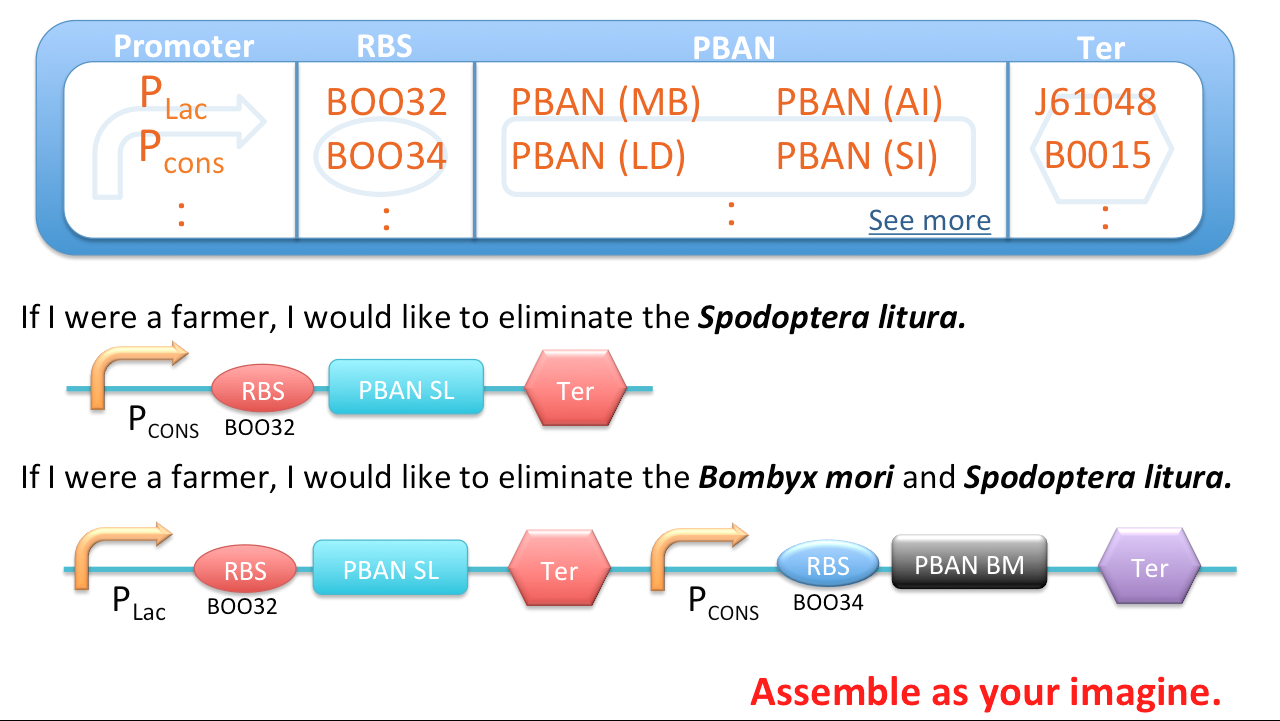
We searched the DNA sequences of the PBANs from many kinds of insects on NCBI, then contrasted to the amino sequences from papers so that we can select the DNA fragments which directly correspond to gland-stimulating function. By ligating the constitutive promoter (J23101), ribosome binding site (B0034) and PBAN DNA sequence with a terminator (J61048) at the last (we delimit this sequence as basic part), we were able to make E.coli directly produce these PBANs continusely instead of the original complex process of PBAN biosynthesis in insects.
Best Potential of Our PBAN Biobrick - Multifunctional
We can assemble these basic parts together because the base pairs of these basic parts are small. Therefore, we can assemble different basic parts which contain different PBAN DNA sequences to resolve different pest problems. Therefore, our biobrick design can be customized to different pest problems. For instance, there is a farm damaged by 3 kinds of moths : Lymantria dispar, Spodoptera litura and Mamestra brassicae. What we only have to do is ligate the PBAN DNA sequence of 3 basic parts into one plasmid and let the E.coli to express these PBAN for us. After these three kinds of moth ingest these three kinds of PBANs. Subsequently, these 3 species of moths will produce their own pheromones to attract the same species. Brief to say, our PBAN basic part can assemble together with any combination method in infinite possibility.
For much more creative ideas, each PBAN basic part can use different promoter, RBS and terminator to make many different regulation. Thus, our PBAN basic part can not only assemble together but also make different regulations ( use different promoters or RBS ) on each PBAN gene. Thus, our PBAN biobrick really have infinite potential!
Device
Introduction
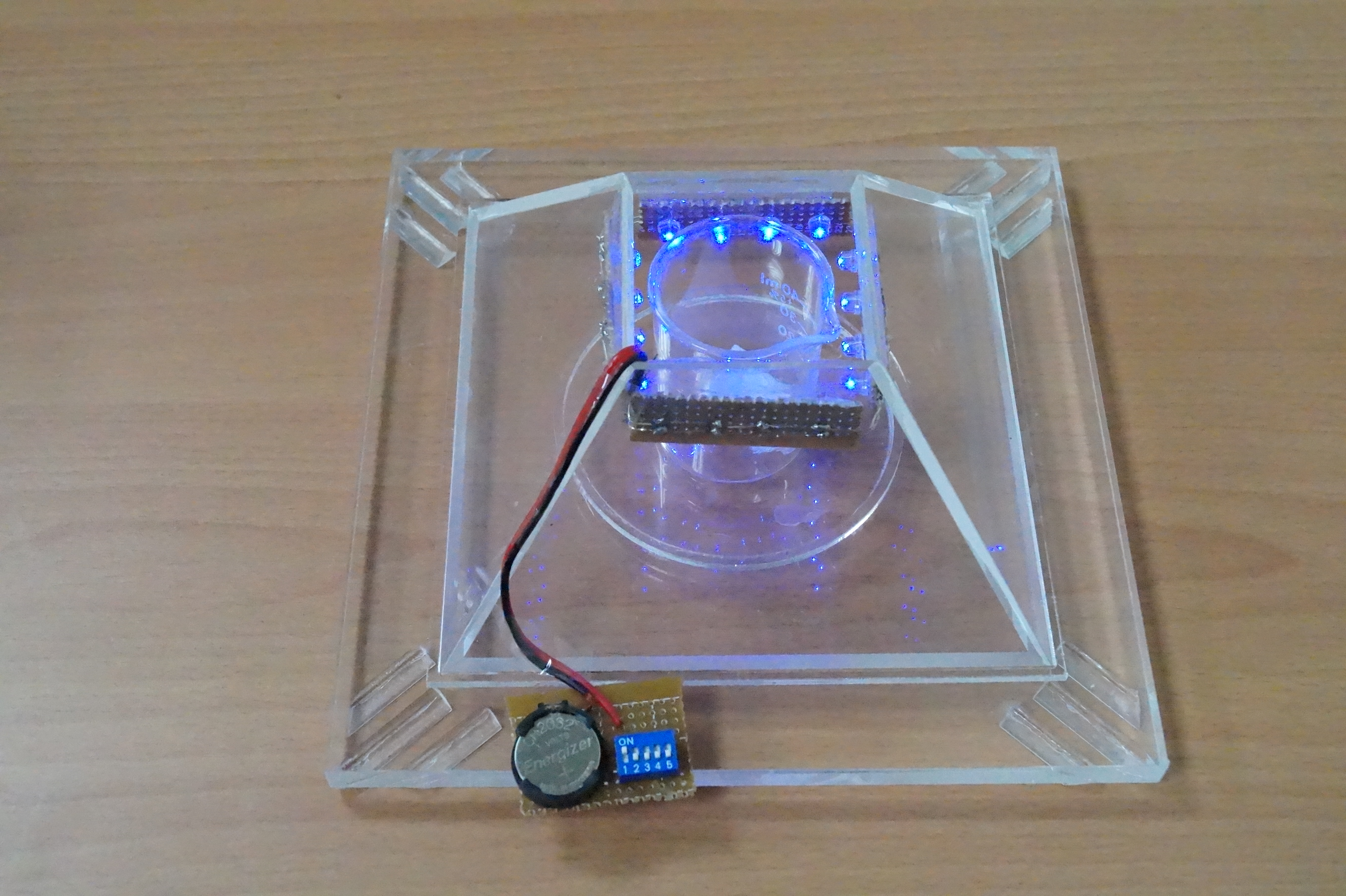
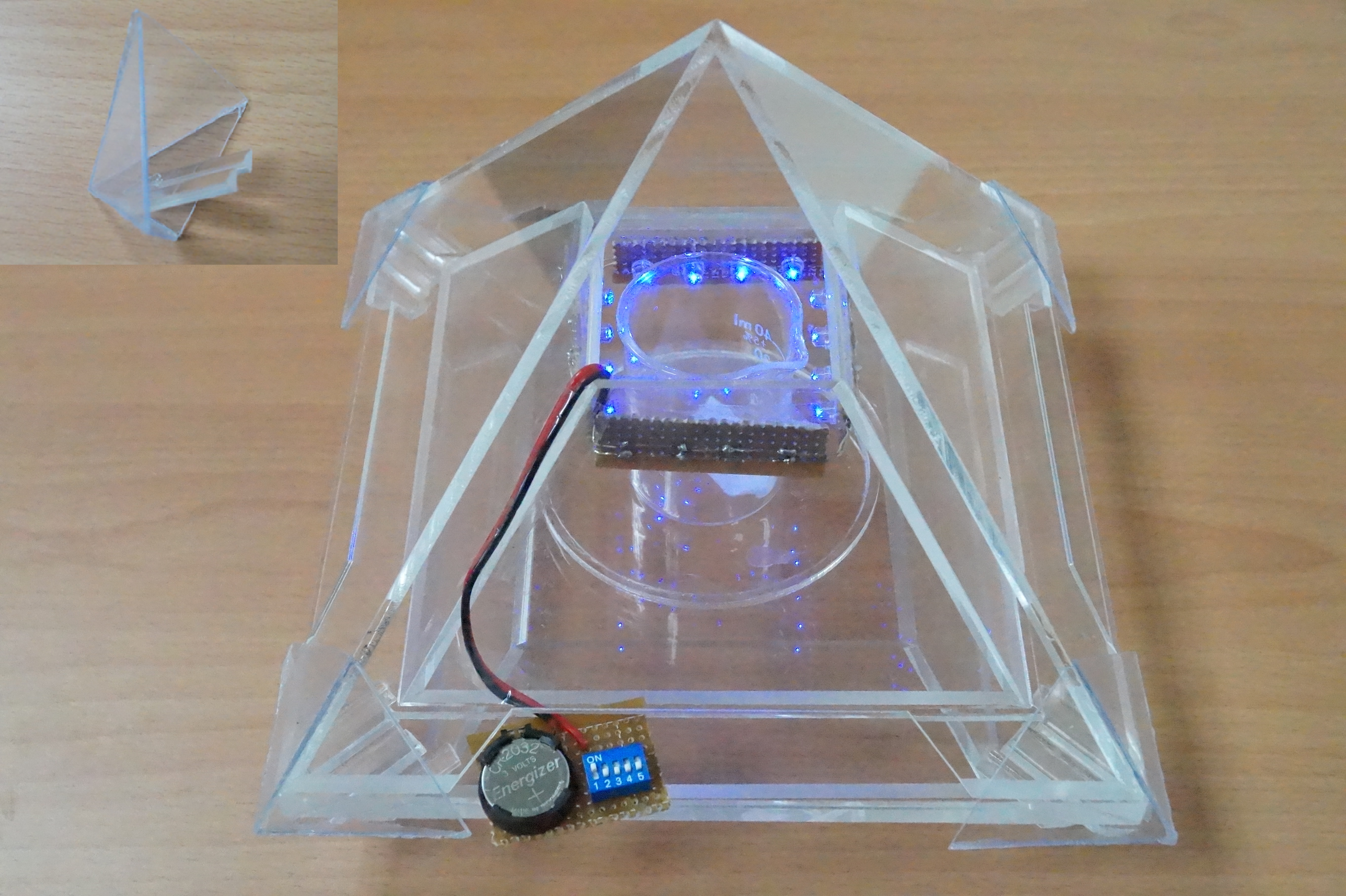
Since insects behave widely different to the gravity force, we design a device which could catch specific kind of insect species that we want. For example, Agrotis ypsilon Rottemberg and Spodoptera litura falls into the kind of moth that have negative geotaxis. So we made a trap with accessible pathway in the bottom. Once an insect enters the device, it could only goes up and be trapped inside the pyramid. However, after field investigation, we found some insects still escape from the device. Then we came out with a new version trap with doubled layers, inner shell and outer shell.
Assembling Process
Before the detail description of how we design our device, we can show you a simple animation of the assembling process of our device. As you can see, our device is very easy to use. Just assemble the outer shell, inner shell ( containing PBAN ) and tenon together and we can complete our device.Then, you can put the device to any where you want to attract the harmful insects.
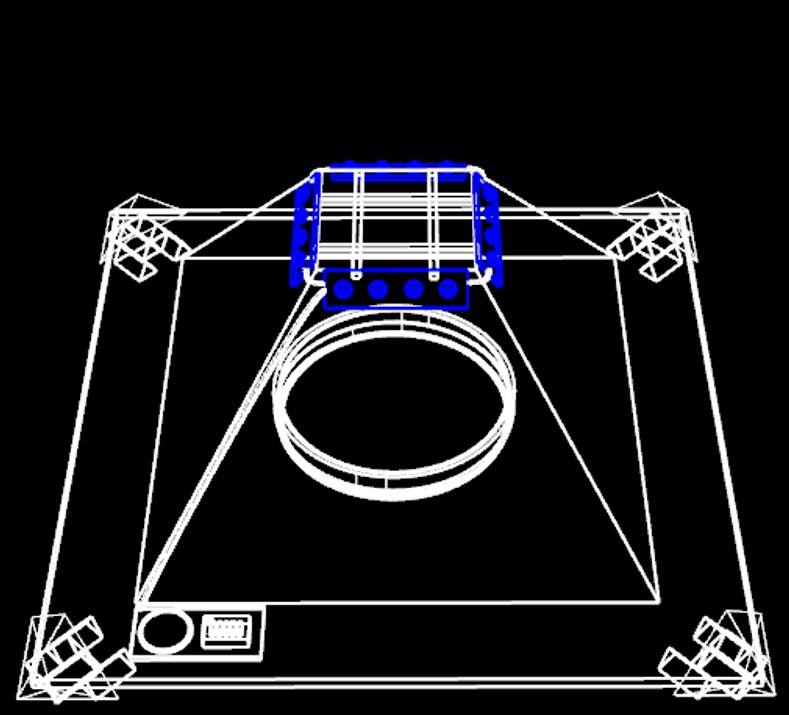
Mechanism
As you can see, we divide our design chart into two parts-exterior and interior. The exterior is just like the appearance of pyramid, and the interior is used to equip PBAN. At first, we turn on the blue light to attract the target harmful insects. When the target female insects we want to catch eat our PBAN, they will release pheromone, and attract the same species. Even we turn off the blue light, our device can attract many target male insects because the female insects inside our device is still in rut and release sex pheromone to attract their mates. After the harmful insects go into our device, the design of our device will take advantages of their characteristic. Insects always fly high to escape so they will be stuck in our device.
Device Design
More detail information about how we design our pyramidal device can download the file below.
-
Device_Design_Download
Process to Assemble Our Device & Main Idea of Our Device Design
Outer Shell:
We shaped the device into a pyramid. Its special layout also enriches the device with mysterious colors.
1. Outer shell is composed of 4 triangular acrylic planes which has a trapezoid entrance to let bugs in and release the smell of pheromone produced by the insects which eat our PBAN.
Inner Shell:
Similar to the outer one, inner shell are also composed of 4 trapezoid planes and removable from the base. The only different part is that its top is not sealed in order to contain the captured insects.
1. There would be a PBAN solution placed on the bottom.
2. A blue LED light bulb will be installed around the top of the inner shell plane to attract the first female insect.
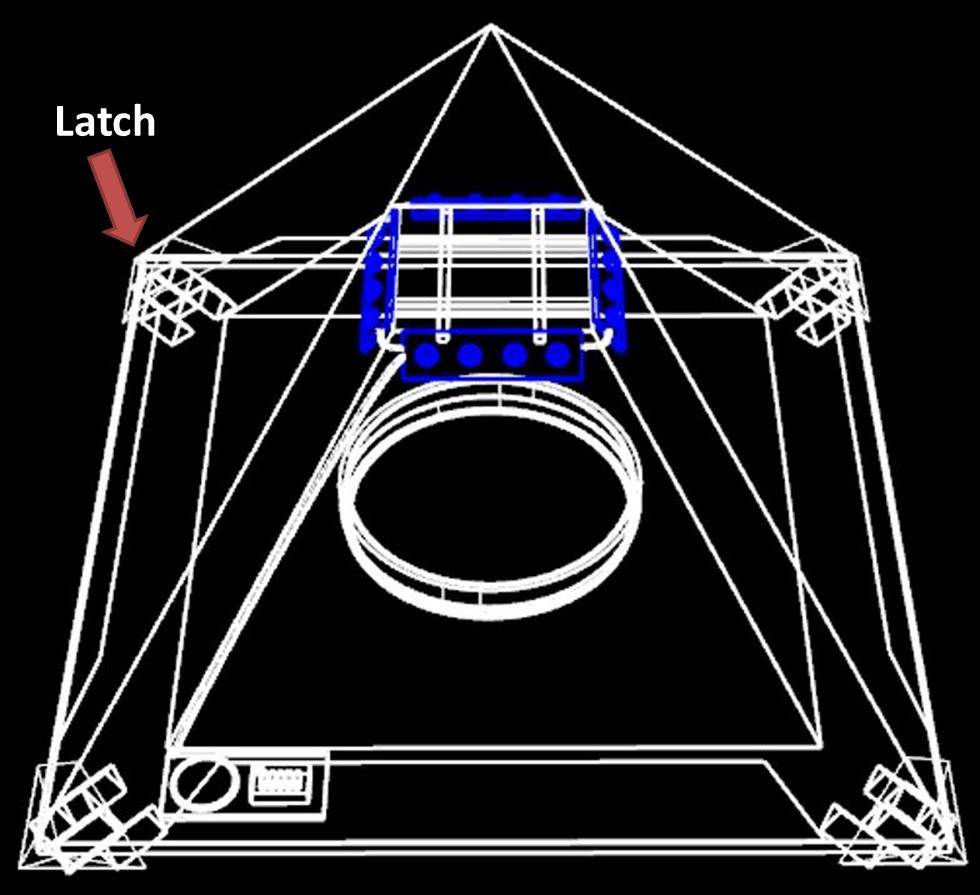
Latch:
A part to stabilize the pyramid.
1. Stabilize 4 corners of the bottom.
2. To make sure the outer shell can combine with the inner one tightly.
Advantages
1. We successfully apply the geotaxis of targeted female moth to trap them in our device, forcing them releasing sex
pheromone by our PBAN to attract more same-kind insects.
2. The hook attached to the purse-string bag can seal it simultaneously when we want to remove the outer shell. By doing so, no insects would flee from the bag and safety problems can also be solved.
3. Inner shell is removable so it’s easier to add new PBAN solution.
4. Compared to conventional light bulb, LED bulb is much brighter and conserves more energy. It could powered by battery so it’s also easier to establish.
5. Purse-string bag is cheap and easy to switch.
6. The PBAN system can run day and night. Its function won’t be affected by sunlight.
7. Pyramid is good at looking and can enriches the entire device with a technological feeling.
 "
"