Team:NCTU Formosa/project
From 2014.igem.org
(→Process to Assemble Our Device & Main Idea of Our Device Design) |
(→Process to Assemble Our Device & Main Idea of Our Device Design) |
||
| Line 277: | Line 277: | ||
<br> | <br> | ||
'''Outer Shell:''' | '''Outer Shell:''' | ||
| - | [[File:Outer_Shell.JPG|350px|thumb|left|fig.1-4-3]][[File:Outer_Shell_1.JPG| | + | [[File:Outer_Shell.JPG|350px|thumb|left|fig.1-4-3]][[File:Outer_Shell_1.JPG|270px|center|thumb|fig.1-4-4]] |
<p> | <p> | ||
We shaped the device into a pyramid. Its special layout also enriches the device with mysterious colors. </p> | We shaped the device into a pyramid. Its special layout also enriches the device with mysterious colors. </p> | ||
Revision as of 14:38, 12 October 2014
- Overview
- PBAN
- Biobrick
-
Overview
Insect damage has been a serious problem for a long time over the whole world and it is difficult to be solved. People have took several methods to kill these harmful insects, but the methods have caused other problems to the environment. In order to solve these troublesome problems and not to cause side effect, we established an alternative way to solve it in an eco-friendly manner.
-
PBAN
PBAN (Pheromone Biosynthesis Activating Neuropeptide) is a simple, short peptide. When it binds with a receptor on the pheromone gland of an insect, it will activates pheromone synthesis. Just like the pheromones, PBAN is species-specific, so we can use one kind of PBAN to enhance pheromone biosynthesis on the kind of insect that we are targeting.
-
Biobrick
In our basic design, we want to express PBAN in an easier way than the original complex process. To make it more convenient to observe and model, we ligate the reporter gene BFP into our biobrick.
Contents
|
Motivation
Serious Problem in The World ( Insect Damage )
From ancient times to the present, harmful insects always play roles in our life, and we don’t have any appropriate solution to these annoying pests. Every year, harmful insects cause a great amount of loss to our agriculture, which seriously disturbs our people livelihood and economy.
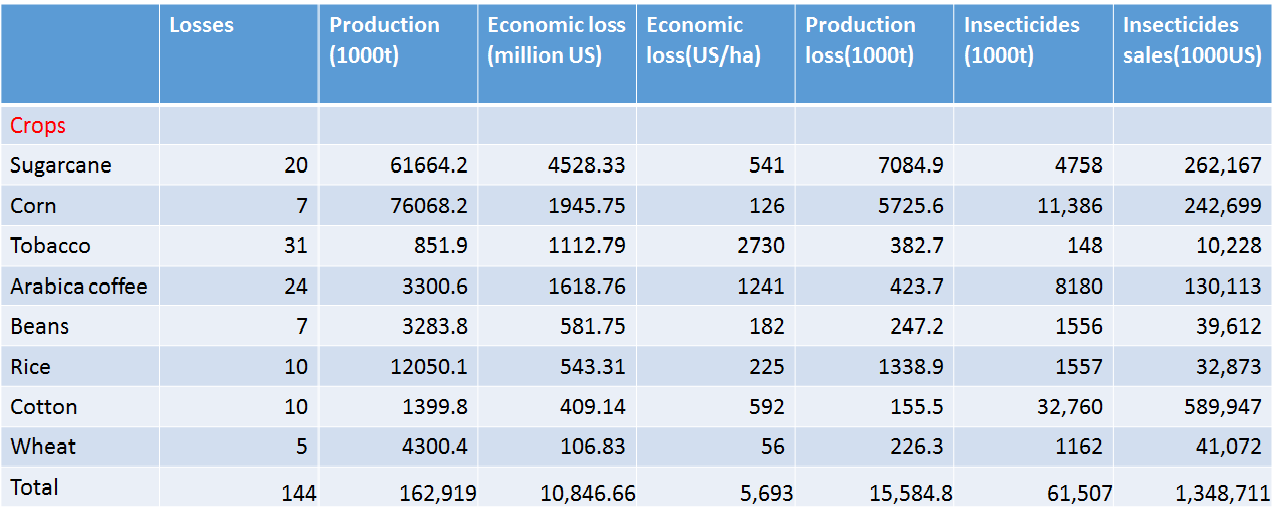
Take Brazil for example. We can find several crucial economic crops such as coconut, coffee, and sugarcane are damaged by harmful insects. The total loss of crops in Brazil is about 11 billion US dollars. In addition, we can also find that people spent nearly 1.4 billion controlling these annoying pests. What's more? They have used a great number of insecticides containing some toxic substances. However, this method will make human beings exposed to horrible situation.
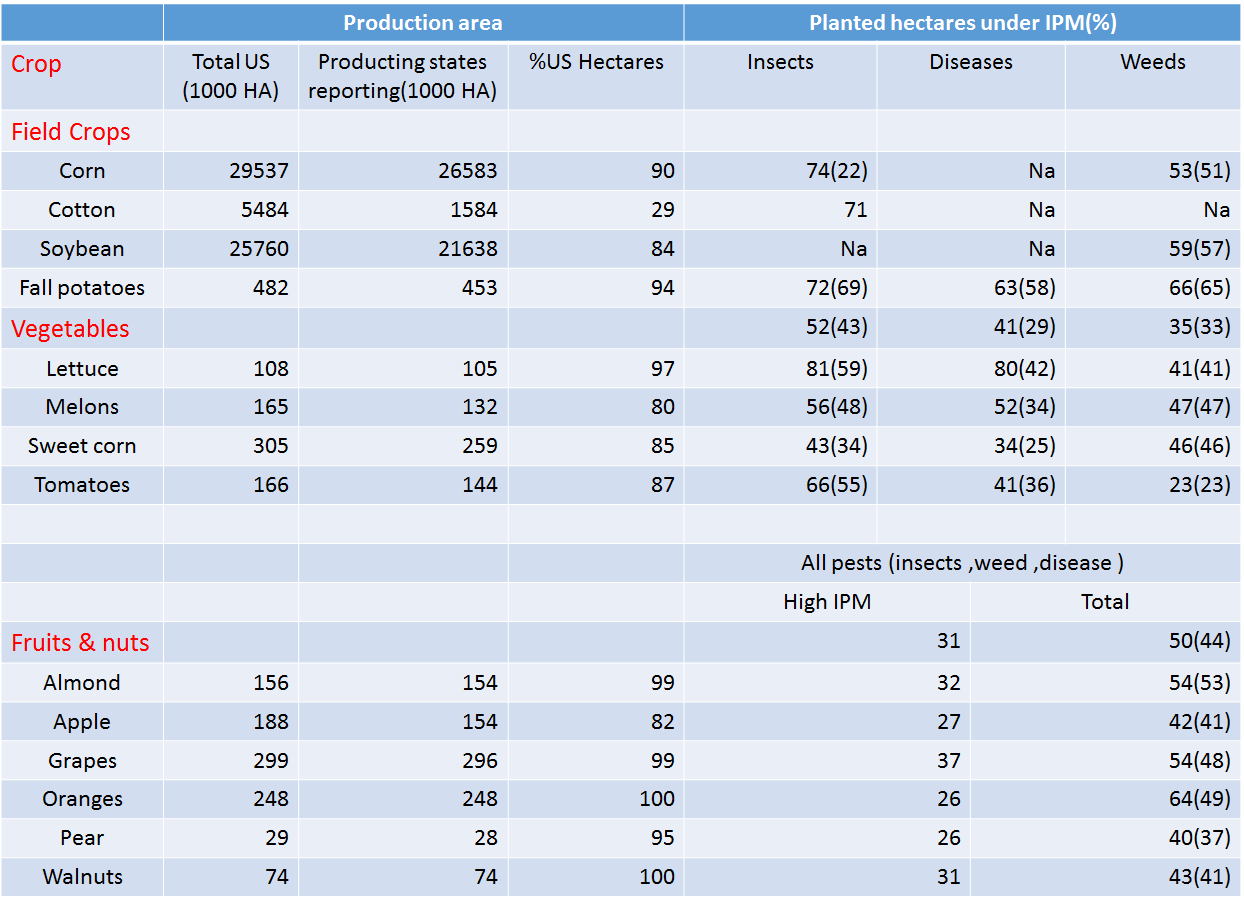
Also, there is a data about the IPM (Integrated pest management) of USA. IPM means the ability to control pests. The IPM is reality number in global, and the lower of the number is, the more efficiently we control. In the data, we can see that lots of areas in USA are used to plant crops. There are several kinds of plants which are still under attack by harmful insects. We can only control the insect damage fairly well in a small limited production area. Therefore, as the production areas expand to an extent, the effect of control declines to a point. Even though the production areas become larger, the total production is lower.
Therefore, what we need is invent a brand new method to solve this problem.
Common Methods of Solving Insect Damage Problem
Chemical Control Method
Because of the hazard of insects, human beings have come up with lots of ideas to kill these harmful insects. In the 15th century, people used heavy metals such as Arsenic, Mercury and Plumbum to kill harmful insects, which caused a catastrophe to the environment. Pesticides became more powerful along with the technology, in the 20th century, the agriculture developed rapidly just because of the evolution of pesticides. But the pesticides are not only fatal to the insects but also harmful to the human beings. People found this problem after several decades. The toxin of the pesticides will be kept in creatures by the food chain, and finally, goes into human's body.
Damage to Human Body
Pesticides includes substances that kill weeds (herbicides), insects (insecticides), fungus (fungicides), rodents (rodenticides), and others. The use of toxic pesticides to manage pest problems has become a common practice around the world. Pesticides are used almost everywhere, causing human health hazards, ranging from short-term impacts such as headaches and nausea to chronic impacts like cancer and reproductive harm. There is also mounting evidence that exposure to pesticides disrupts the endocrine system, wreaking havoc with the complex regulation of hormones, the reproductive system, and embryonic development.
Damage to The Environment
Pesticides wreak havoc on the environment, threatening biodiversity and weakening the natural systems upon which human survival depends. Pesticides have led to abnormal mass mortality of America's honeybees. The populations of bees have dropped by 29% to 36% each year since 2006. To our astonishment, fully 1/3 of the food we eat depends on bees for pollination!
Secondly, some scientists believe that amphibians and bats have become more susceptible to deadly disease because their immune systems are weakened by pesticides. What's more, one kind of pesticides called herbicide, which can give a male frog a sex change. Genetically, the frogs are still males, but morphologically they are completely female and they can even mate successfully with other males and lay viable eggs. Pesticides have also contaminated waterways and endangered fish and birds, causing ecological damage.
By the way, pesticide resistance makes insecticides ineffective. Other factors in the speed with which a species evolves resistance are generation time and fecundity, that is, causing shorter generations and more offspring lead to resistance more quickly.
Biological Control Method
Since most chemical control methods are poisonous to the environment, humans began to look for other alternative ways. One of the most well known example is bacillus thuringiensis (Bt), which can efficiently kill insects with its crystal proteins. However, recent researches have shown that more and more insects have been resistant to this kind of protein.
Chemical control method have caused fetal environmental damge and more and more harmful insects can be resistant to it. Also, biological bacillus thuringiensis (Bt) control method can be resisted by more and more insects. However, it's not too late to improve this situation. We can create an evolution of agriculture by a new method, and that is what we do!
Pheromone trap is the novelist method to solve the insect damage problem nowadays. What's more, pheromone is a pollution-free substance and a substance of insect's itself. Thus, pheromone doesn't have any insect's resistance problem. Thus, we initially took biological synthesis of pheromone in E.coli into consideration.
Novel and Efficient Ways to Solve Insect Damage Problem - Pheromone Trap
Introduction of Pheromones
A pheromone is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting outside the body of the secreting individual to impact the behavior of the receiving individual.
There are many kinds of pheromones such as alarm pheromones, food trail pheromones, sex pheromones, and many others that affect behavior or physiology.
Sex pheromones are an important factor in finding a mating partner. When a female releases chemicals, the mate search is initiated, and the male moths begin their upwind motion toward their potential partner. Sex pheromones in particular are associated with long-range chemical communication of sex substances used in signaling a mating partner. Mate finding in moths involve sex pheromones that have the ability to propel long-distances and are emitted by the females abdominal glands in most cases.
How to Make Pheromones?
There are 2 main ways to make pheromones. One is chemical synthesis, the other one is biosynthesis approach.
Biosynthesis Pathway in Insects
Biosynthesis approach needs lots of enzymes to catalyzed, which is too complicated for E.coli to carry out. In addition, E.coli is lack of Glycoproteins that can do posttranslational modification. Therefore, it is nearly impossible for E.coli to produce pheromones in a normal way.
Chemical Synthesis Approach Used in Factory to Mass Produce Pheromone
It is difficult to make pheromone by chemical synthesis approach. This approach needs to use many kinds of chemical compound which may cause some pollution to the environment. Furthermore, chemical approach needs to control the condition of reaction that may consume a lot of energy. An example of chemical synthesis approach: leafroller moth, Bonagota cranaodes.
Difficulty and Disadvantage of Pheromone Production
As above mentioned, because pheromone is a very complicated substance, it absolutely has many problems to produce it. Speaking of chemistry production, though pheromone can be mass produced to save the cost, chemistry production still has many derived problems, like high producing cost ( difficult and very complex technique needed to control many reaction conditions ) and most importantly, serious environmental pollution ( using many chemical reagents to purify and produce pheromone ). Thus, it is no mentioned to produce pheromone in biosynthesis method with E.coli. Therefore, we decided to find a more easy and better way to reach our goal.
Idealist Method of Solving Insect Damage Problem - PBAN
Though, it is impossible to biosynthesize pheromone with E.coli, we still tried our best to find another more feasible way. As above mentioned, our method should be pollution-free, efficient, insects' resistance-free and cheap. Conclude all these advantages elements, and we found PBAN, which can help us let target harmful insects produce pheromone for us. Moreover, we even can use biosynthesis method to make our project multifunctional. We hope we can use PBAN to solve the target harmful insects below. ( Check photos )
-
Cabbage MothMamestra brassicae
-
Cotton bollwormHelicoverpa armigera (Hubner)
-
Oriental Leafworm MothSpodoptera litura
-
Red imported fire antSolenopsis invicta
-
Yellow fever mosquitoAedes (Stegomyia) aegypyi
-
Black cutwormAgrotis ipsilon
-
Gypsy MothLymantria dispar
-
LeafrollersStatherotis leucaspis Meyrick
-
SilkwormBombyx mori
Spread:This moths has a natural range across Europe, Asia, and North Africa.
Characteristics:The larva is green, khaki, grey-brown or brown with dark spots(1)(2)(3). The topside is darker than the bottom side and a yellow or light brown stripe goes round the middle portion by the spots.
Damage:The caterpillar of this species is seen as a pest for commercial agriculture. Often referred to as the "imported cabbageworm" they are a serious pest to cabbage and other mustard family crops. It can also be a pest of cultivated brassicas and sweet peas, but it feeds on a wide range of other plants.
Control: Organic controls(4) for cabbage worms include handpicking, excluding them with row cover barriers, or treating with a Bt pesticide. Cabbage worms are found throughout North America, and more than one species may be found in the same garden.
Spread:The pink bollworm has spread to cotton-growing regions throughout the world.
Characteristics: The larva is green, khaki, grey-brown or brown with dark spots(5). The topside is darker than the bottom side and a yellow or light brown stripe goes round the middle portion by the spots(32).
Damage: The cotton bollworm is a highly polyphagous species.[6] The most important crop hosts are tomato, cotton, pigeon pea, chickpea, sorghum and cowpea. Other hosts include groundnut, okra, peas, field beans, soybeans, lucerne, Phaseolus spp., other Leguminosae, tobacco, potatoes, maize, flax, Dianthus, Rosa, Pelargonium, Chrysanthemum, Lavandula angustifolia, a number of fruit trees, forest trees and a range of vegetable crops(6).
Control: Cultural controls, with the exception of the use of Bt cotton and the use of mating disruption and sprays of the Entrust formulation of spinosad are acceptable to use on organically grown cotton(7).
Spread:Widely distributed in Asia and Oceania.
Asia: Afghanistan, Bangladesh, Cambodia, China, Hong Kong, Indonesia, India, Iran, Japan, Laos, Malaysia, Myanmar, Nepal, North Korea, Oman, Pakistan, Philippines, Singapore, South Korea, Sri Lanka, Taiwan, Thailand, Vietnam. Oceania: Australia, Guam, New Caledonia, New Zealand, Micronesia, Papua New Guinea,
Samoa, other Pacific islands. United States: Hawaii(8).Characteristics: Adult moths measure between 15-20 mm (0.59-0.79 inches) in length and have a wingspan of 30-38 mm (1.18-1.5 inches). Forewings are gray to reddish-brown, with a complex pattern of creamy streaks and paler lines along the veins. Hind wings are grayish-white with grayish-brown margins. Males have a blue-grey band from the upper corner (apex) to the inner margin of each forewing. Larvae have bright yellow stripes along the back and the sides. Larval color varies from pale green to dark green(9)(31).
Damage: Oriental Leafworm Moth Spodoptera litura is a Noctuid moth which is considered as an agricultural pest. It is also known as the Cluster caterpillar, Cotton leafworm, Tobacco cutworm, and Tropical armyworm. It has a very wide host range of over 120 plant species, including: lettuce, cabbage, beetroot, peanuts, geranium, cotton, banana, fuchsias, acacia, African oil palm, amaranth, alfalfa, strawberry, sorghum, sugarcane, tomatoes, asparagus, apple, eggplant, beet, beans, broccoli, elephants ear, horsetail she oak, corn, flax, lantana, papaya, orange, mango, leek, among many others.
Control: The use of Bacillus thuringiensis (BT) may effectively control this pest. Other forms of biological, horticultural, and cultural control that have been studied include: planting near derris and garlic plants, breeding resistant plants from wild plants for example groundnuts from wild groundnuts, breeding resistant plants using bacterium Bacillus thuringiensis genes, using a Baculovirus, using the nematode Steinernema carpocapsae, and using the fly Exorista japonica(10).
Spread:The red imported fire ant, a eusocial species, are far more aggressive than most ant species. Animals, including humans, often encounter them by inadvertently stepping on one of their mounds, which causes the ants to swarm up the legs, attacking en masse. The ants respond to pheromones released by the first ant that attacks, thereafter stinging in concert(12).
Characteristics: Fire ants are red and black in coloration and, like all insects, they are protected by a hard exoskeleton and have six legs. Worker ants have round heads with mandibles, an armored thorax midsection and an abdomen, made up of the pedicle and the gaster. The head is typically copper brown in color. In addition to their mandibles, fire ant workers also possess an abdominal stinger(11)(29).
Damage: They are considered to be a pest, not only because of the physical pain they can inflict, but also because their mound-building activity can damage plant roots, lead to loss of crops(12).
Control: Hot water Pouring hot water on the mounds is effective and environmentally friendly, but may require 3 or 4 applications to kill the colony. Water should be at least scalding hot, but does not need to be boiling. This works best when you use 3 to 4 gallons of water in each application. WARNING: Hot water kills grass and shrubbery and may cause severe burns if spilled. Liquid nitrogen: it could be the most effective and most environmental friendly method to eradicate the species(30)(13).
Spread:The yellow fever mosquito, Aedes aegypti, is a mosquito that can spread the dengue fever, chikungunya, and yellow fever viruses, and other diseases. The mosquito can be recognized by white markings on its legs and a marking in the form of a lyre on the thorax. The mosquito originated in Africa, but is now found in tropical and subtropical regions throughout the world(14).
Characteristics: The mosquito can be recognized by white markings on its legs and a marking in the form of a lyre on the thorax.
Damage: The yellow fever mosquito, Aedes aegypti, is a mosquito that can spread the dengue fever, chikungunya, and yellow fever viruses, and other diseases(15).
Control: Empty water from containers such as flower pots, birdbaths, pet water dishes, cans, gutters, tires and buckets regularly to disrupt the mosquito breeding cycle. Consider using an insect repellent, be sure to follow the label directions for applying the repellent. For help selecting a mosquito repellent, try our Insect Repellent Locator(16).
Spread: This Caterpillar can be found, as various species, through be serious foliage feeders on some crops such as peanuts.hout North America.
Characteristics: Cutworms common in Georgia fields are black (Agrotis ipsilon (Ashmed)), granulate (Agrotis subterranea (Fabricius)) and variegated cutworm (Peridroma saucia(Hubner)). These are moths in the family Noctuidae. Full-grown cutworm larvae are 1.5 to 2 inches long. Coloration will vary among species, but all tend to be stout-bodied caterpillars with four sets of prolegs. They have the tendency to curl into a ball when disturbed(17).
Damage: Almost any plant can be attacked in the seedling stage. Cotton and certain vegetables sometimes have stand reductions(17).
Control: Bacillus thuringiensis, a widely available caterpillar-killing bacterium,is a very effective control for climbing cutworms as well as for the surface feeders(18).
Spread: It has a range which covers Europe, Africa, and North America(19).
Characteristics:Gypsy moth caterpillars change appearance as they grow. Young caterpillars are black or brown and about ¼ inch (0.6 cm) in length. As they grow, bumps develop along their backs along with coarse, black hairs. Each of the 11 sections of a developed caterpillar will have two coloured spots, the first five pairs, blue, and the last six, red. Mature caterpillars can be as long as 2 ½ inches (6.35 cm)(20).
Damage: It is classified as a pest, and its larvae consume the leaves of over 500 species of trees, shrubs and plants. The gypsy moth is one of the most destructive pests of hardwood trees in the eastern United States.he gypsy moth was considered a nuisance just ten years after their release. It included an account of all the trees being defoliated, caterpillars covering houses and sidewalks and that the caterpillars would rain down upon residents. The first outbreak occurred in 1889. An eradication program was begun in 1890.
Control: Tanglefoot Pest Barrier or Sticky Tree Bands can be placed around tree trunks to help curtail the caterpillars movement into and out of the tree canopy. Apply Bacillus thuringiensis, var. kurstaki or Monterey Garden Insect Spray (Spinosad) to the leaves of trees to kill gypsy moth caterpillars(21).
Spread: The obliquebanded leafroller (OBLR) is native to and widely distributed throughout temperate North America(22).
Characteristics: hatched larvae have a yellowish green body and a black head and thoracic shield. Mature larvae are 20 to 25 mm in length and the head and thoracic shield may be either black or various shades of brown(23).
Damage: Leafrollers, the larvae of certain tortricid moths, often feed and pupate within the protection of rolled-up leaves. Several species can cause problems on fruit and ornamental trees in California. The fruittree leafroller, Archips argyrospila, is the most common leafroller pest in landscapes throughout the state. It occurs on many ornamental trees—including ash, birch, California buckeye, box elder, elm, locust, maple, poplar, rose, and willow—and is particularly damaging to deciduous and live oaks. It also attacks numerous fruit and nut trees including almond, apple, apricot, caneberries, cherry, citrus, pear, plum, prune, quince, and walnut(24).
Control: Several parasites attack OBLR larvae but do not adequately control the pest. Apply sprays during June to kill the first summer brood adults and newly hatching larvae(24).
Spread: the place where farmers want to feed.
Characteristics : It is entirely dependent on humans for its reproduction and does not occur naturally in the wild(26).
Damage: None
Control: Not necessary
Reference
-
1.Pogue, Michael. "A review of selected species of Lymantria Huber [1819]". Forest Health Technology Enterprise Team. Retrieved September 14, 2012.
2.Eliminate Cutworms Using Natural Pest Control By Susan Glaese March/April 1987
3. Laurence Mousson, Catherine Dauga, Thomas Garrigues, Francis Schaffner, Marie Vazeille & Anna-Bella Failloux (August 2005). "Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations". Genetics Research
4. NPIC is a cooperative agreement between Oregon State University
5.M. S. Ascunce et al., “Global Invasion History of the Fire Ant Solenopsis invicta”, Science, vol. 331, no. 6020, pp. 1066 - 1068, 2011
6.organicgardening.com/learn-and-grow/fire-ant-control
7.Scientists suggest fighting fire ants with ice By Chiu Yu-Tzu / STAFF REPORTER
8.Capinera, J.L. 1999. Fall armyworm Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae
9.Helicoverpa Diapause Induction and Emergence Tool Introduction to the Helicoverpa armigera Genome Project Helicoverpa armigera Genome Project updates on InsectaCentral Helicoverpa Genome Project database on-line Lepiforum Funet Taxonomy Fauna Europaea
UC IPM Pest Management Guidelines: Cotton UC ANR Publication 3444
10. "Interesting (To Us) Photos From The Garden". Meades.org. Retrieved 2011-08-11.RXwildlife Sightings » Blog Archive » More Emergence". Rxwildlife.org.uk. 2009-06-03. Retrieved 2011-08-11.Cabbage Moth - Caterpillar". Habitas.org.uk. Retrieved 2014-08-11.
11.Authored by W. H. Reissig. Published by the New York State Agricultural Experiment Station, Geneva, A Division of the New York State College of Agriculture and Life Sciences, A Statutory College of the State University, Cornell University, Ithaca. Funded in part by an Extension Service-USDA, IPM Grant.
PBAN ( Pheromone Biosynthesis Activating Neuropeptide )
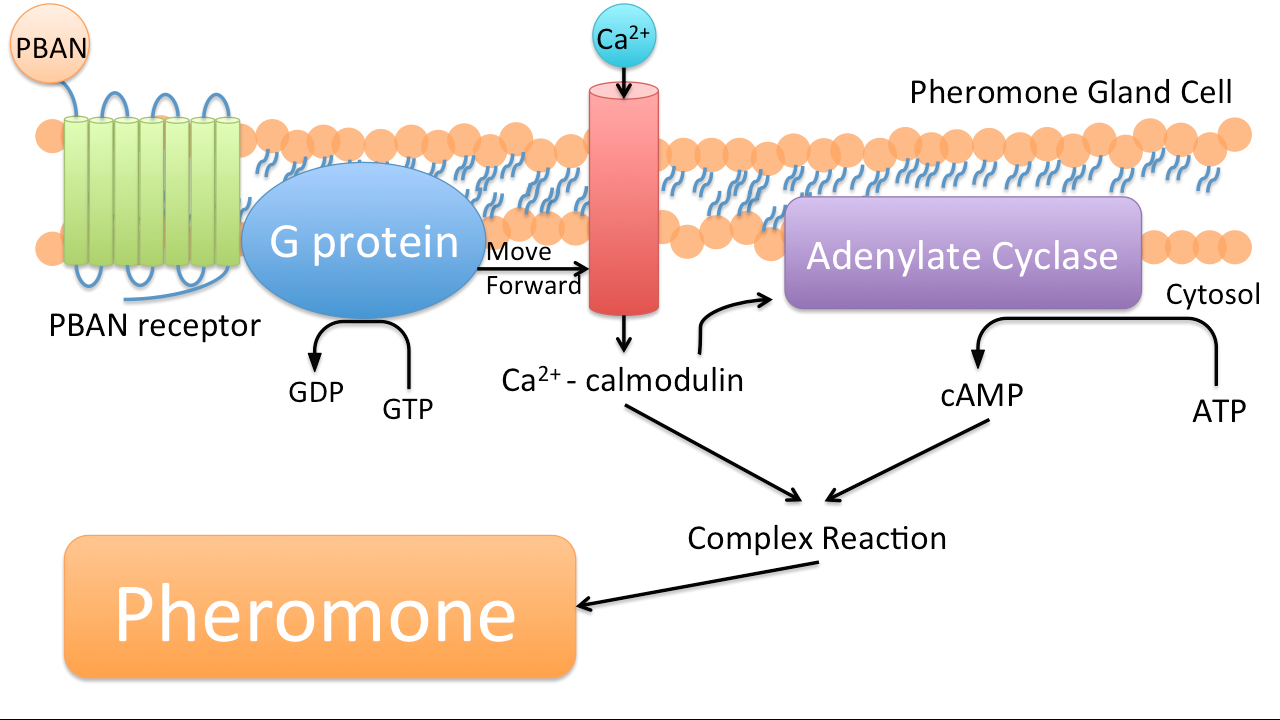
PBAN (Pheromone Biosynthesis Activating Neuropeptide) is one kind of peptides that can activate biosynthesis of pheromones of insects we target. Once a PBAN binds with the G-protein coupled receptor on an insect’s pheromone gland, the signal sent by the G-protein coupled receptor activates the kinase and phosphatase, and then kinase and phosphatase can activate enzymes that participate in the biosynthesis of insect sex pheromone, which will be emitted.
In nature, female insects such as moths release PBAN during mating to stimulate the synthesis of pheromones in order to attract their male counterparts. PBAN can also facilitate the release of non-sex pheromones such as trail pheromones for ants.
Features of PBAN
1. Species-Specific:PBAN is species-specific just like pheromones, that means every kinds of insects which can produce pheromone have it's specific PBAN, which can only bind with it's specific receptor and only stimulate the biosynthesis of a specific pheromone.
2. Small Simple Peptide:The coding sequence for a PBAN is usually around 100 base pairs. Therefore, it is easy for E.coli to express. Thus, all we have to do is to cultivate the E.coli to express our PBAN so our cost is very low. In addition, we can even combine several different kinds of PBAN sequences into one BioBrick assembly or even make different regulations for different PBANs without DNA length problem. Last but not least, PBAN is a peptide unlike pesticide so it dosen't cause any damage to the environment.
3. Natural Substance of Insects' Itself: Because PBAN is a natural substance of insects' itself, insects have no possibility to resist it. Thus, our PBAN unlike pesticide and bacillus thuringiensis (Bt) can cause target insects to continuously produce pheromone for us if it can come in contact to the pheromone gland.
Effect Testing of PBAN from Reference
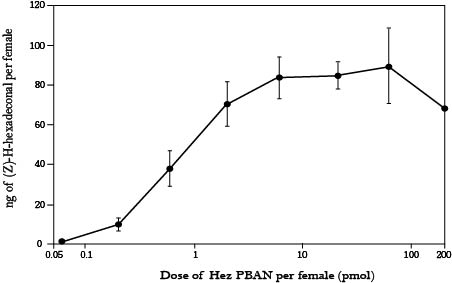
Because we regard PBAN as a leading character in our project, we have to realize what kind of problem we would meet with if we want to make use of PBAN. First, we had to confirm that whether PBAN can work when getting in the moth’s body from outside, we got a paper titled “Pheromonotropic Activity of Naturally Occurring Pyrokinin Insect Neuropeptides (FXPRLamide) in Helicoverpa zea” from ELSEVIER, experiments of the research were conducted with Helicoverpa zea (corn earworm) in this research, researchers injected different amount (from 0.05 pmol to 200 pmol) of PBAN of Helicoverpa zea (named Hez-PBAN) into their bodies directly, and then measured the amount of pheromone produced, the result (fig.1-2-4) showed that PBAN has function as long as it gets in moth’s body, and the 2 pmol dose of Hez-PBAN was the minimum dose required to stimulate production of the maximum amount of pheromone, that revealed we are able to stimulate the production of pheromone with only little amount of PBAN which gets in moth’s body, whether we fed or injected PBAN.
Difficulty of Natural PBAN about Peptidase Degradation
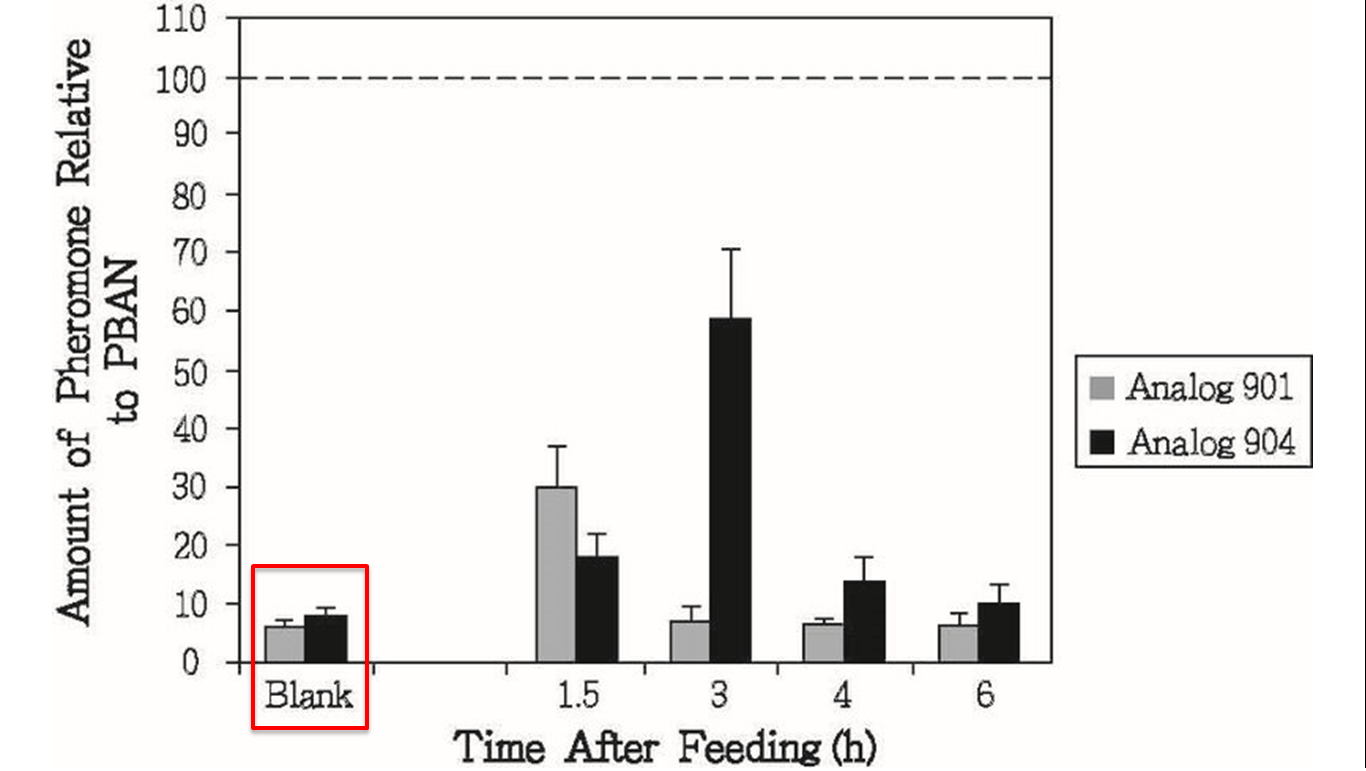
But we still had the second problem, we had known that peptidase in the insect’s body can degrade peptides including PBAN, so the effect of PBAN can’t continue for a long time. In order to solve this problem, we search some former papers and we found another paper from ELSEVIER, it was titled “Enhanced oral availability/pheromonotropic activity of peptidase-resistanttopical amphiphilic analogs of pyrokinin/PBAN insect neuropeptides”, Heliothis virescens was used in this research, researchers produced amphiphilic analogs of pyrokinin/PBAN with chemical synthesis method, they used hydroxyproline (Hyp) and octahydroindole-2-carboxyl (Oic) to replace the Proline (Pro) of the PBAN C-terminal penpapeptides (Phe-Thr-Pro-Arg-Leu-NH2), that made PBAN be able to resist peptidase, they compared the stability of the natural pyrokinin/PBAN analog LPK, and the peptidase-resistant pyrokinin/PBAN analogs (5 nmol was used), we can see that natural PBAN will be reduced over time in the moth’s body (fig.1-2-5).
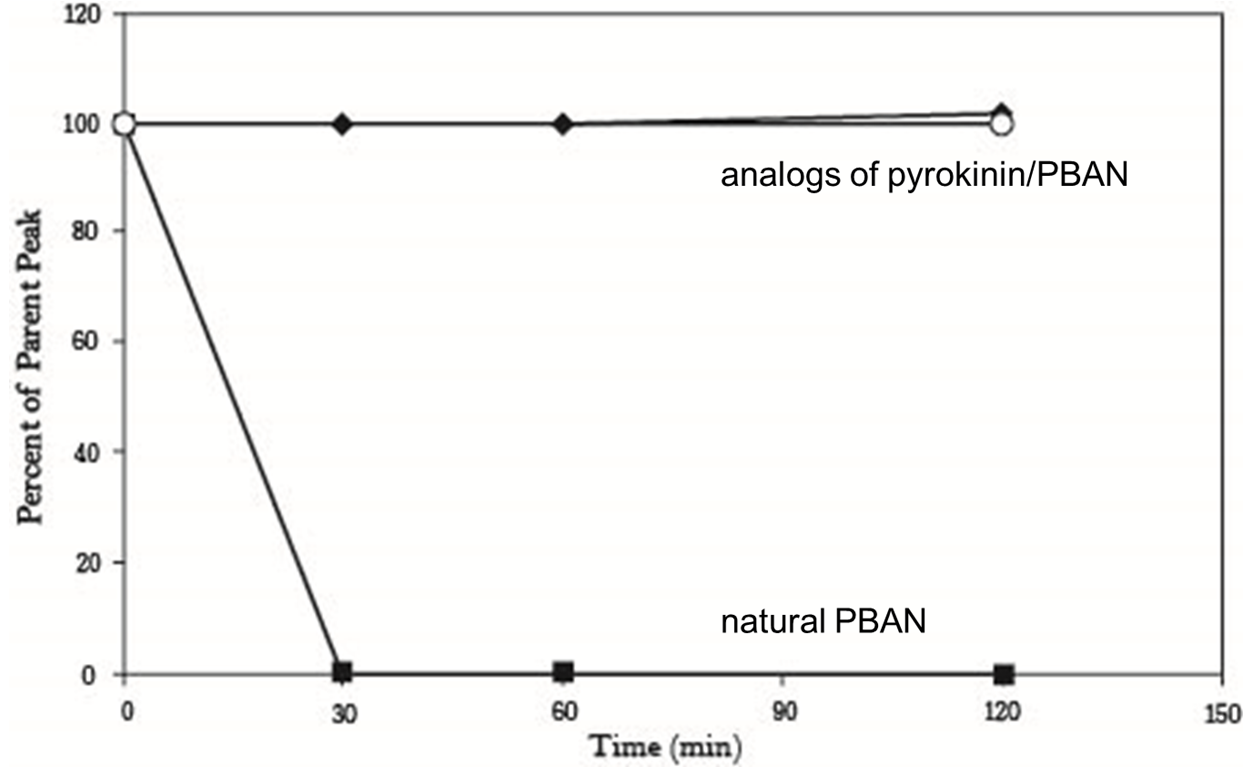
Our New Idea of Using PBAN from Oral Testing in Refernece
This results above sounded not good for us, but there was other experiment interested us in the same research, researchers also conducted oral test with their PBAN (fig.1-2-6), in this experiment, they fed moths with sugar solution which contained their PBAN analogs, and measure the amount of pheromone produced over time, that gave us inspiration, although natural PBAN can’t be maintained for a long time in moth’s body, we could solve this problem by feeding moths with PBAN continually, after combining this thought with the method of the oral experiment, we figured out an idea, instead of killing all of the moths.
It is very possible that the PBAN in the food will penetrate into moths’ body from the digestive system. Thus, if female moths suck the food, PBAN in the moths’ body will be replenished, which solves the problem that PBAN can not maintain for a long time in the moths’body. As to how we produce PBAN and apply our concept to capture wanted harmful insects, we will explain in the following.
How We Are Going to Use PBAN?
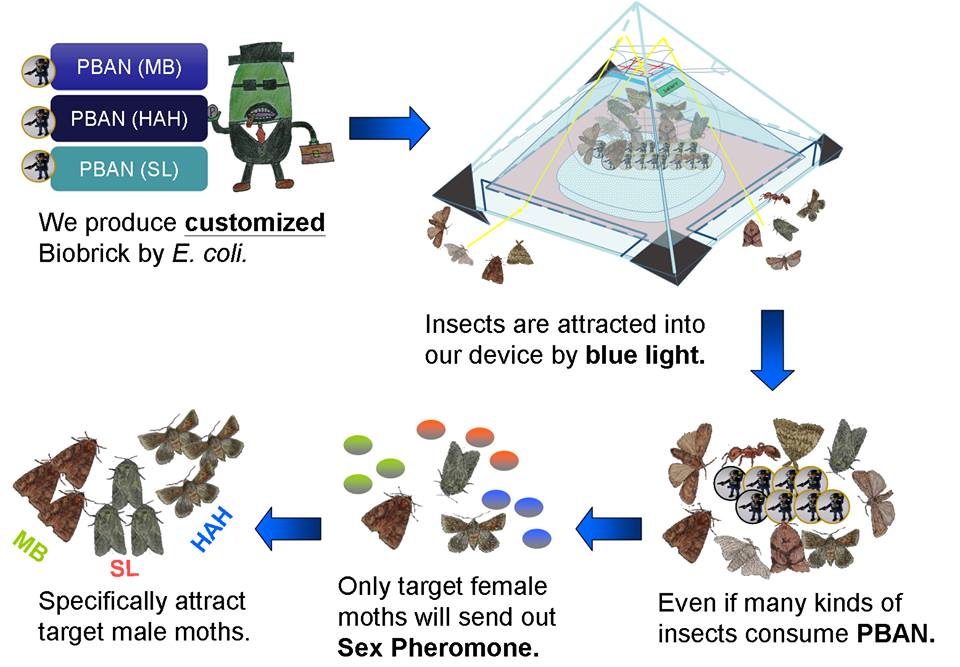
In our project, we will biologically synthesize PBAN with our E.coli. We store the PBAN inside a trapping device (check this out at our Device page). In the device, there will be appropriate lighting and nutrient sources that will attract insects.
Once an insect is attracted into our device and ingests the nutrient sources we provide, it will also inevitably come in contact with our PBAN. As the PBAN works its magic and activates the pheromone synthesis of the attracted insect, more of this species of insect’s counterparts will be attracted and later captured.
Owing to the first feature mentioned above, PBAN are species-specific, which means that it doesn't matter if other kind of insect fly into our device and eat PBAN, because the insects we don't want to catch will not be stimulated by PBANs to produce pheromone; our PBAN are only for what we want to catch, and we are sure that our method won't affect other kinds of insects.
Biobrick Design
Basic Biobrick Design to Use Our PBAN
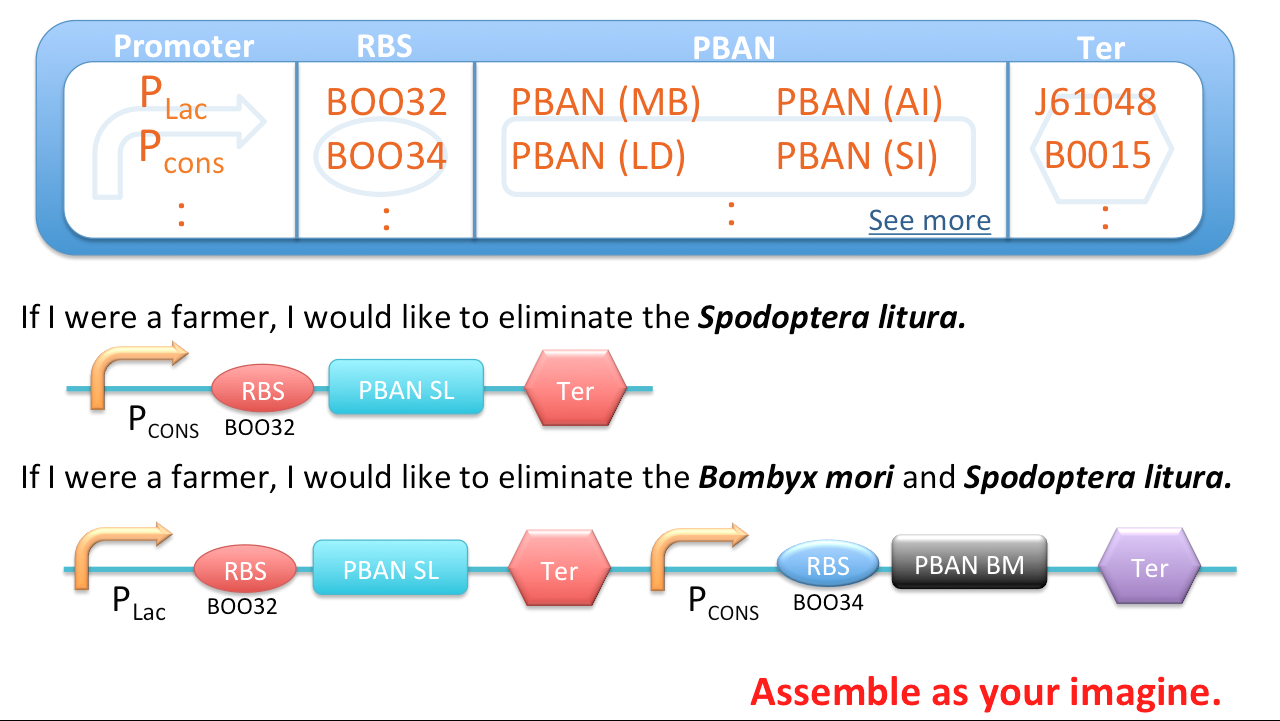
We searched the DNA sequences of the PBANs from many kinds of insects on NCBI, then contrasted to the amino sequences from papers so that we can select the DNA fragments which directly correspond to gland-stimulating function. By ligating the constitutive promoter (J23101), ribosome binding site (B0034) and PBAN DNA sequence with a terminator (J61048) at the last (we delimit this sequence as basic part), we were able to make E.coli directly produce these PBANs continusely instead of the original complex process of PBAN biosynthesis in insects.
Best Potential of Our PBAN Biobrick - Multifunctional
We can assemble these basic parts together because the base pairs of these basic parts are small. Therefore, we can assemble different basic parts which contain different PBAN DNA sequences to resolve different pest problems. Therefore, our biobrick design can be customized to different pest problems. For instance, there is a farm damaged by 3 kinds of moths : Lymantria dispar, Spodoptera litura and Mamestra brassicae. What we only have to do is ligate the PBAN DNA sequence of 3 basic parts into one plasmid and let the E.coli to express these PBAN for us. After these three kinds of moth ingest these three kinds of PBANs. Subsequently, these 3 species of moths will produce their own pheromones to attract the same species. Brief to say, our PBAN basic part can assemble together with any combination method in infinite possibility.
For much more creative ideas, each PBAN basic part can use different promoter, RBS and terminator to make many different regulation. Thus, our PBAN basic part can not only assemble together but also make different regulations ( use different promoters or RBS ) on each PBAN gene. Thus, our PBAN biobrick really have infinite potential!- Multifunction
Reference
- Torsten Waldminghaus, Nadja Heidrich, Sabine Brantl and Franz Narberhaus .(2007). FourU: a novel type of RNA thermometer in Salmonella . Molecular Microbiology , 65(2): 413–424 DOI:10.1111/j.1365-2958.2007.05794.x
- part BBa_K115002;TUDelft Registry of Standard Biological Parts
Device
Introduction
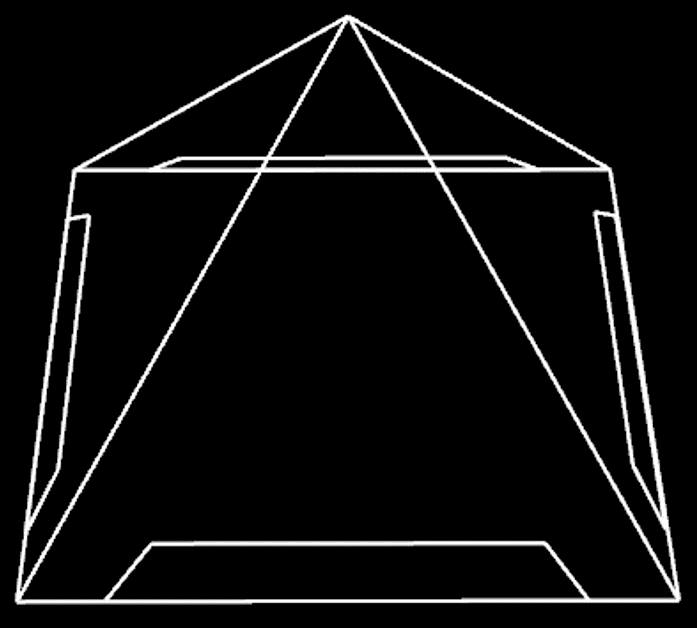
Since insects behave widely different to the gravity force, we design a device which could catch specific kind of insect species that we want. For example, Agrotis ypsilon Rottemberg and Spodoptera litura falls into the kind of moth that have negative geotaxis. So we made a trap with accessible pathway in the bottom. Once an insect enters the device, it could only goes up and be trapped inside the pyramid. However, after field investigation, we found some insects still escape from the device. Then we came out with a new version trap with doubled layers, inner shell and outer shell.
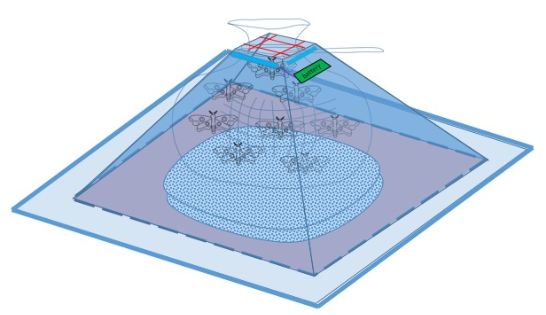
Mechanism
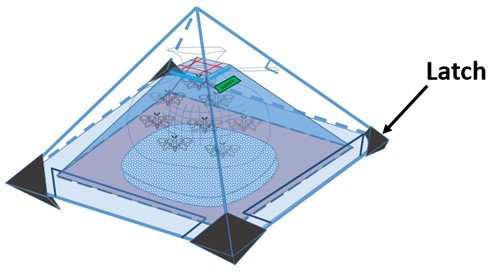
First, we divide our design chart into two parts-exterior and interior. The exterior is just like the appearance of pyramid, and the interior is used to equip PBAN and bag for pests. When the harmful insects we want to catch eat our PBAN, they will release pheromone, and attract the same species. We can use blue light or the smell of pheromone to attract insect at first. After they go into our device, the design of our device will take advantages of their characteristic that insects always fly high to escape and make them stuck in our device. When we take away the outer shield, the hock on the outer shield will close the bag, and the insects will be caught. In addition, the four tenons at the corner can firm up our device.
Device Design
More detail information about how we design our pyramidal device can download the file below.
-
Device_Design_Download
Process to Assemble Our Device & Main Idea of Our Device Design
Outer Shell:
We shaped the device into a pyramid. Its special layout also enriches the device with mysterious colors.
1. Outer shell is composed of 4 triangular acrylic planes which has a trapezoid entrance to let bugs in and release the smell of pheromone produced by the insects which eat our PBAN.
Inner Shell:
1. There would be a PBAN solution placed on the bottom.
2. A blue LED light bulb will be installed around the top of the inner shell plane to attract the first female insect.
Tenon:
A part to stabilize the pyramid.
1. Stabilize 4 corners of the bottom.
2. To make sure the outer shell can combine with the inner one tightly.
Assembling Process
We can assemble the outer shell、inner shell and tenon together to get our completed pyramid device.
Advantages
1.We successfully apply the geotaxis of targeted female moth to trap them in our device, forcing them releasing sex pheromone by our PBAN to attract more same-kind insects.
2.The hook attached to the purse-string bag can seal it simultaneously when we want to remove the outer shell. By doing so, no insects would flee from the bag and safety problems can also be solved.
3.Inner shell is removable so it’s easier to add new PBAN solution.
4.Compared to conventional light bulb, LED bulb is much brighter and conserves more energy. It could powered by battery so it’s also easier to establish.
5.Purse-string bag is cheap and easy to switch.
6.The PBAN system can run day and night. Its function won’t be affected by sunlight.
7.Pyramid is good at looking and can enriches the entire device with a technological feeling.
 "
"