Team:ETH Zurich/lab/bead
From 2014.igem.org
(→Production) |
(→Overview) |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
| - | + | [[File:ETH2014_BeadLogo.jpg|left|300px]] | |
Alginates are present as structural components in both the cell walls of brown algae and the capsules of soil bacteria. However, commercially available alginate is mainly extracted from algae. The polysaccharides find a broad application in various fields: in textile printing, in food industry or in research. The chemical features of alginates allow immobilization of macromolecules and cells, thus, the compound is commonly used in biotechnology, biomedicine and pharmacy<sup>[[Team:ETH_Zurich/project/references#refEmergence|[24]]]</sup>. In our project we encapsulated bacteria in alginate beads so as to ensure local separation of the different units (here strains) and directional communication between them. These prerequisites are required for controlled pattern formation. | Alginates are present as structural components in both the cell walls of brown algae and the capsules of soil bacteria. However, commercially available alginate is mainly extracted from algae. The polysaccharides find a broad application in various fields: in textile printing, in food industry or in research. The chemical features of alginates allow immobilization of macromolecules and cells, thus, the compound is commonly used in biotechnology, biomedicine and pharmacy<sup>[[Team:ETH_Zurich/project/references#refEmergence|[24]]]</sup>. In our project we encapsulated bacteria in alginate beads so as to ensure local separation of the different units (here strains) and directional communication between them. These prerequisites are required for controlled pattern formation. | ||
| Line 15: | Line 15: | ||
===Properties=== | ===Properties=== | ||
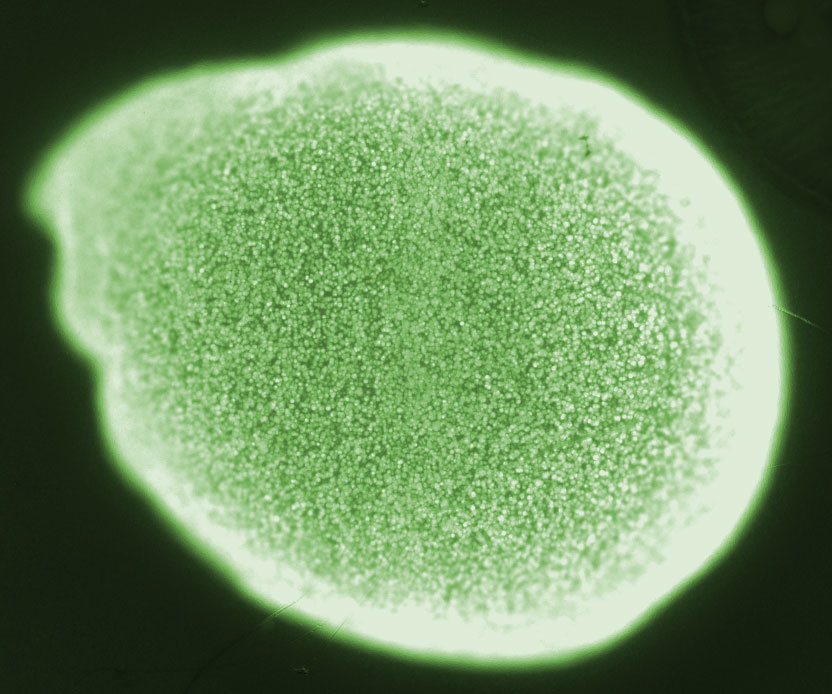
| + | [[File:ETH2014_BeadColonies.jpg|left|300px]] | ||
Na<sup>2+</sup> alginate is a viscous liquid, however, upon addition multivalent ions such as Ca<sup>2+</sup> cross-linking of the polysaccharides occurs. Thus gelling of alginate can be achieved by the addition of a Ca<sup>2+</sup>. For encapsulation the cells or macromolecules are added to Na<sup>2+</sup> alginate and subsequently immobilized during the gelling process. In fact, the encapsulation process is mild and compatible with most living cells. The high porosity of the ionically cross-linked polysaccharide lattice allows diffusion of nutrients and other substances into and out of the bead. This property of Ca<sup>2+</sup> alginate allows cultivation of bacteria inside beads and does not prevent communication via small molecules between colonies of different beads. Substances such as phosphate or EDTA are sequestrating Ca<sup>2+</sup> and thus destabilizing the alginate gel<sup>[[Team:ETH_Zurich/project/references#refEmergence|[25]]]</sup>. This fact should be considered when choosing the cultivation medium. | Na<sup>2+</sup> alginate is a viscous liquid, however, upon addition multivalent ions such as Ca<sup>2+</sup> cross-linking of the polysaccharides occurs. Thus gelling of alginate can be achieved by the addition of a Ca<sup>2+</sup>. For encapsulation the cells or macromolecules are added to Na<sup>2+</sup> alginate and subsequently immobilized during the gelling process. In fact, the encapsulation process is mild and compatible with most living cells. The high porosity of the ionically cross-linked polysaccharide lattice allows diffusion of nutrients and other substances into and out of the bead. This property of Ca<sup>2+</sup> alginate allows cultivation of bacteria inside beads and does not prevent communication via small molecules between colonies of different beads. Substances such as phosphate or EDTA are sequestrating Ca<sup>2+</sup> and thus destabilizing the alginate gel<sup>[[Team:ETH_Zurich/project/references#refEmergence|[25]]]</sup>. This fact should be considered when choosing the cultivation medium. | ||
Revision as of 20:44, 15 October 2014
Beads
Overview
Alginates are present as structural components in both the cell walls of brown algae and the capsules of soil bacteria. However, commercially available alginate is mainly extracted from algae. The polysaccharides find a broad application in various fields: in textile printing, in food industry or in research. The chemical features of alginates allow immobilization of macromolecules and cells, thus, the compound is commonly used in biotechnology, biomedicine and pharmacy[24]. In our project we encapsulated bacteria in alginate beads so as to ensure local separation of the different units (here strains) and directional communication between them. These prerequisites are required for controlled pattern formation.
Properties
Na2+ alginate is a viscous liquid, however, upon addition multivalent ions such as Ca2+ cross-linking of the polysaccharides occurs. Thus gelling of alginate can be achieved by the addition of a Ca2+. For encapsulation the cells or macromolecules are added to Na2+ alginate and subsequently immobilized during the gelling process. In fact, the encapsulation process is mild and compatible with most living cells. The high porosity of the ionically cross-linked polysaccharide lattice allows diffusion of nutrients and other substances into and out of the bead. This property of Ca2+ alginate allows cultivation of bacteria inside beads and does not prevent communication via small molecules between colonies of different beads. Substances such as phosphate or EDTA are sequestrating Ca2+ and thus destabilizing the alginate gel[25]. This fact should be considered when choosing the cultivation medium.
Production
Loading the Chip
 "
"