Team:Austin Texas/kit
From 2014.igem.org
| Line 102: | Line 102: | ||
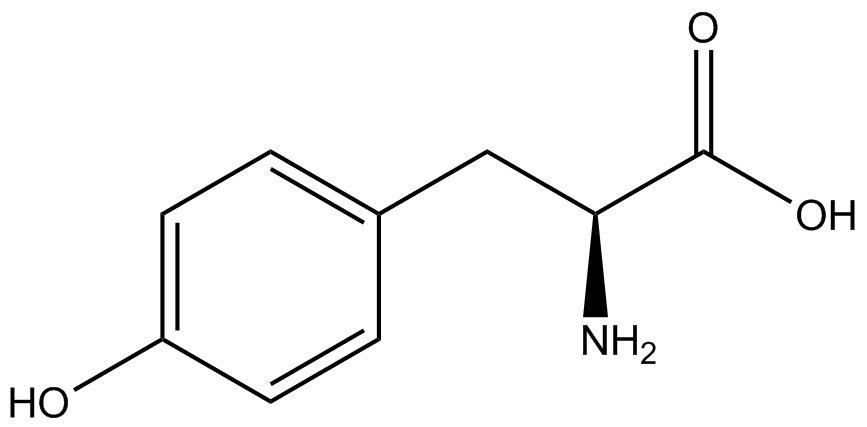
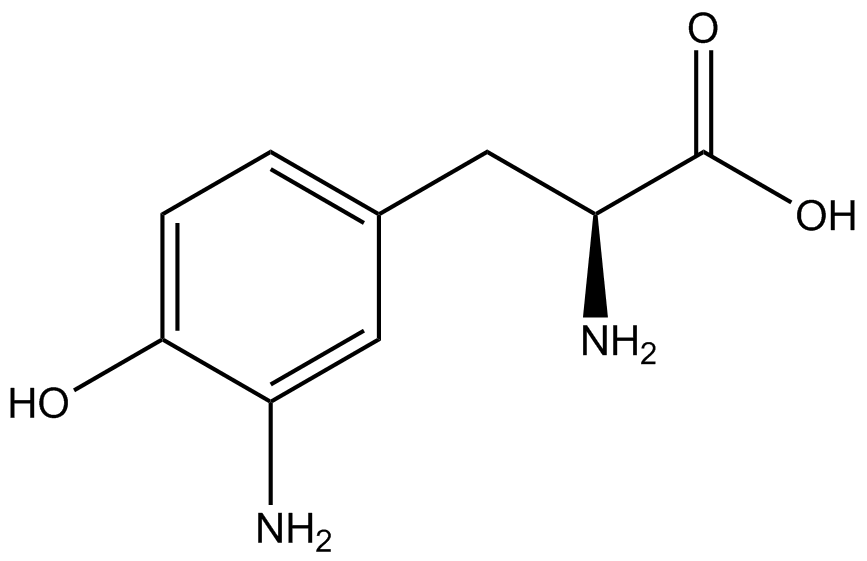
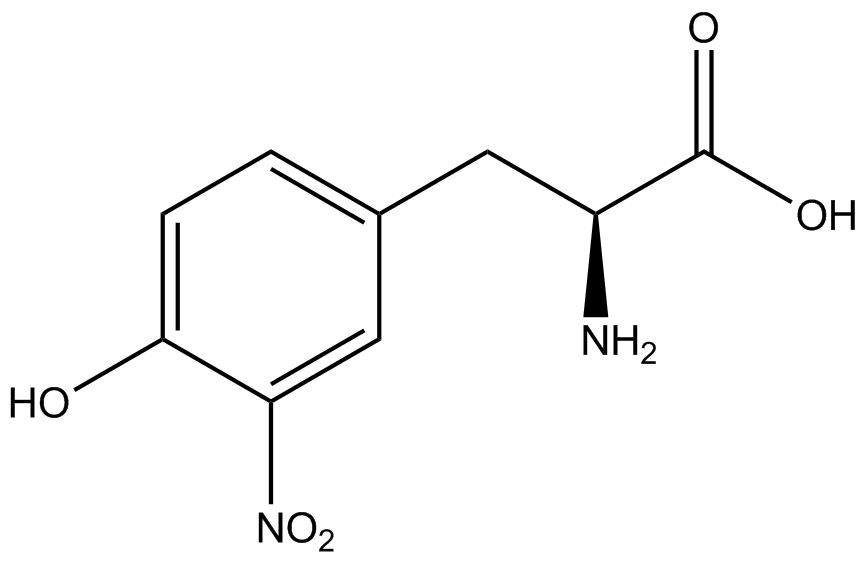
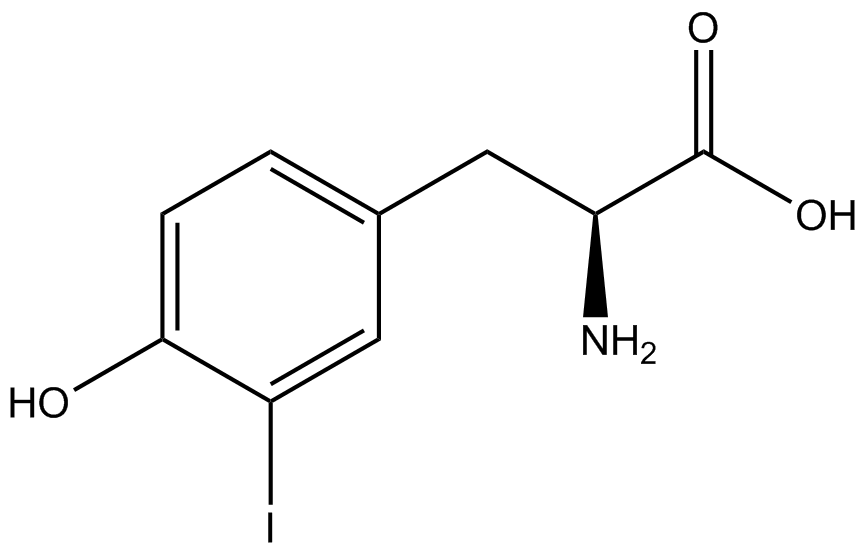
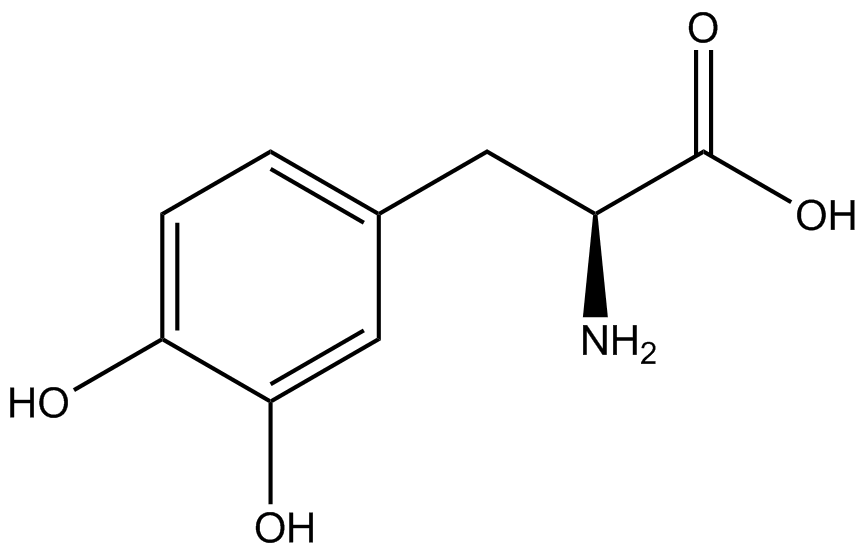
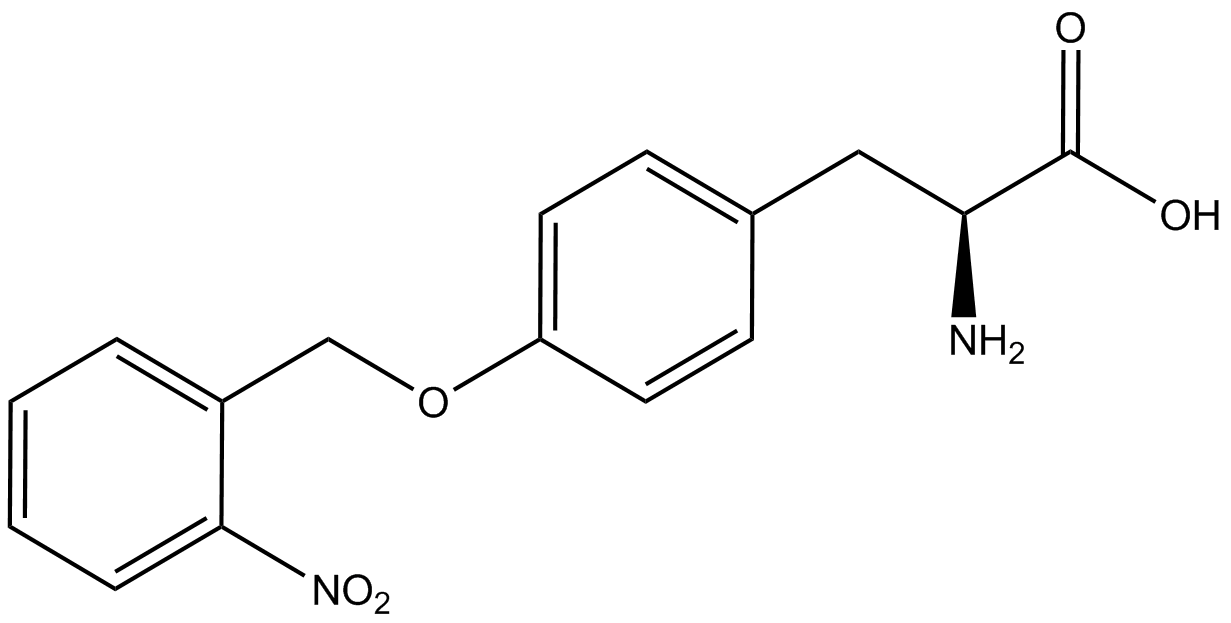
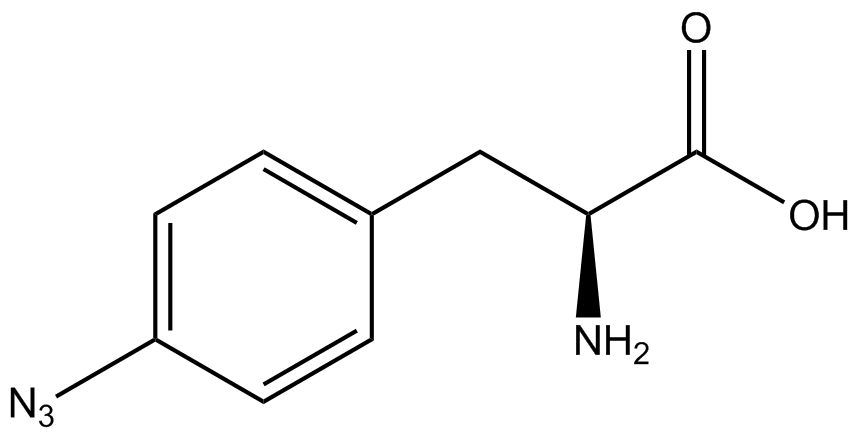
| - | The Schultz lab was the first to expand the genetic code using a unique synthetase/tRNA. This synthetase /tRNA pair originated from M. jannaschii tyrosine RS (Mj-TyrRS) and allowed the cell to incorporate <i>O</i>-methyl-L-tyrosine at the amber codon (Wang et al. 2001). Since then, | + | The Schultz lab was the first to expand the genetic code using a unique synthetase/tRNA pair. This synthetase/tRNA pair originated from M. jannaschii tyrosine RS (Mj-TyrRS) and allowed the cell to incorporate <i>O</i>-methyl-L-tyrosine at the amber codon (Wang et al. 2001). Since then, numerous ncAA synthetase/tRNA pairs have been generated. A total of seven ncAAs were used in addition to tyrosine for our project. [https://2014.igem.org/Team:Austin_Texas/kit#ncAA_Table The full list of amino acids used in this study can be found here]. |
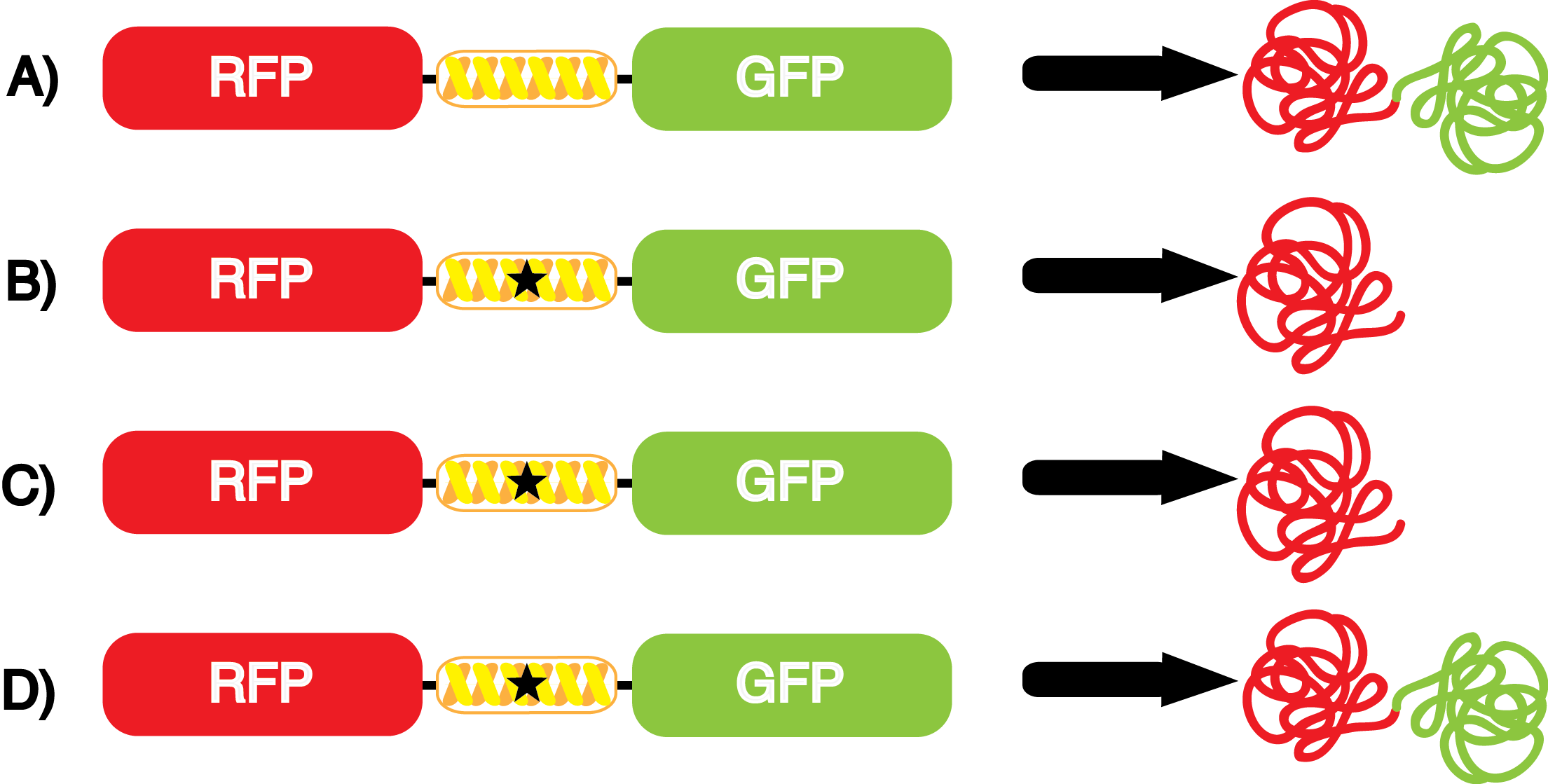
| - | It is important to note that complications can arise when the genetic code is recoded. In a normal bacterium, release factor RF1 is responsible for terminating translation when the ribosome reaches an amber stop codon. To avoid termination at | + | It is important to note that complications can arise when the genetic code is recoded. In a normal bacterium, release factor RF1 is responsible for terminating translation when the ribosome reaches an amber stop codon. To expand the potential of ncAAs, it is vital to avoid termination at amber codons. Seeing this need, the Church and Isaacs groups engineered a strain of ''E. coli'' that had all of the amber codons removed from the genome, which allowed them to knock out the RF1 gene (Isaacs et al. 2011). The resulting strain, called "amberless" ''E. coli'', has NO AMBER CODONS. Thus, to better characterize the synthetase/tRNA pairs, we have employed amberless ''E. coli''. The advantage of this system is that since there are no amber codons in the genome, we cannot accidentally interfere with any cellular processes. Additionally, since RF1 is knocked out, there is no competition between RF1 and the novel synthetase/tRNA pair. These factors allow us to better assess the fidelity and efficiency of ncAA tRNA synthetase/tRNA pairs. |
<h1>Experimental Design and Method</h1> | <h1>Experimental Design and Method</h1> | ||
Revision as of 16:50, 17 October 2014
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"