Team:Heidelberg/pages/PCR 2.0
From 2014.igem.org
Contents |
Introduction
Motivation
The invention of the polymerase chain reaction in 1983 by Kary Mulliy revolutionized the modern world by enabling amplification of DNA in an exponential manner. Further improvements including the use of thermo-stable DNA-polymerases made the method even more efficient, allowing its widespread use in nearly every field of modern diagnostics and research. However, a major part of information is lost when using conventional PCR due to missing transfer of DNA modifications. The most abundant modification is DNA methylation, which is present in all kingdoms of life where it acts as prominent regulator of gene expression. Methylation and other modifications that influence the DNA function without changing its actual sequence are studied in the fast-growing field of epigenetics (greek: epi/επί-over, above, around).
To empower epigenetics, we propose the PCR 2.0 as an easy and efficient way to amplify DNA templates in an exponential manner while maintaining their specific methylation pattern. The pivotal element of this PCR reaction is the use of a heat-stable DNA methyltransferase (DNMT). Although comparable enzymes exist in thermophile organisms [1] until now no suitable protein has been found or synthesized that withstands the harsh conditions of a PCR.
Even though the detection and mapping of methylation patterns has become feasible in a high-throughput manner by using bisulfite sequencing and array techniques [2], further functional analysis is still hindered by the small amount of primary material. Our approach therefore aims to provide unlimited amounts of any methylated DNA sample of interest, without knowledge of methylation patterns or the need of expensive methods such as de-novo synthesis – just by running a PCR.
To achieve this goal of a PCR2.0 that can be used to amplify DNA including its intrinsic methylation pattern, our main aim was the generation of a heat-stable Dnmt1 that can be produced in a larger scale and shows efficient and specific methylation of DNA. Our approach applies protein circularization by using self-splicing protein sequences, socalled inteins from our toolbox as well as our CRAUT Linker Software. Since the restriction of conformational changes through intramolecular bonds has been known to increase the stability of proteins [3] [4] and joining of C- and N- terminus were reported to increases in thermostability of smaller peptides [5] we started the circularization of the biggest protein so far…
Epigenetics - there is more than just A, T, C and G…
In mammals and other vertebrates methylation occurs at cytosine nucleotides that are followed by guanines (CpG) and is a prominent key regulator of transcription, embryonic development, X chromosome inactivation and many other cellular functions. [6] [7]. Approximately 1% of the genetic code in human somatic cells constists of methylated cytosines, amounting to 70-80% of all present CpG dinucleotides [8]. Due to the great prevalence and the heredity of this modification, the 5-methylcytosine (5mC) is also known as the “fifth base” of eukaryotic genomes [9]. DNA methylation is preferentially occurring at intergenic regions and repetitive sequences, where it is known to silence gene expression [10] [11]. In contrast, GC-rich promoter regions are usually characterized by a low methylation status, allowing transcription of the associated genes [12]. Deregulation of cytosine methylation has been reported to play a crucial role in the development of numerous diseases, including cancer, imprinting diseases and repeat-instability based diseases such Huntington's disease [13].
A closer look at DNA Methyltransferases
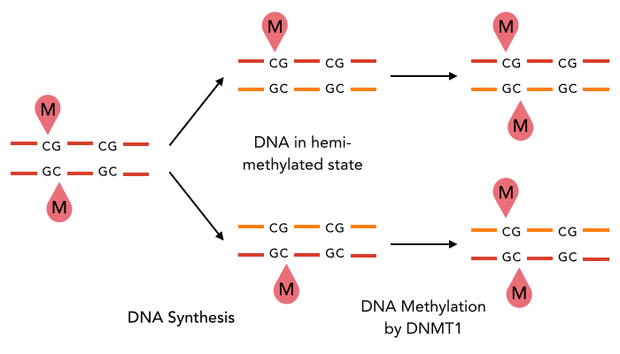
The family of enzymes called DNA methyltransferases (DNMTs) is responsible for the establishment and maintenance of cell-type specific DNA methylation patterns. Whereas the two enzymes DNMT3a and DNMT3b contribute to de novo methylation of DNA during development, DNMT1 is preserving existing methylation patterns throughout cell divisions. To do so, DNMT1 exploits the principle of semiconservative replication, using the parental strand as a template to create an exact copy of methylation patterns on the daughter strand. Subsequent to the recognition of a so called hemi-methylated CpG site, the enzymatic machinery transfers a methyl group from the methyl donor S-Adenosyl-methionine (SAM) to the C5 position of the complementary cytosine residue [15] [19].
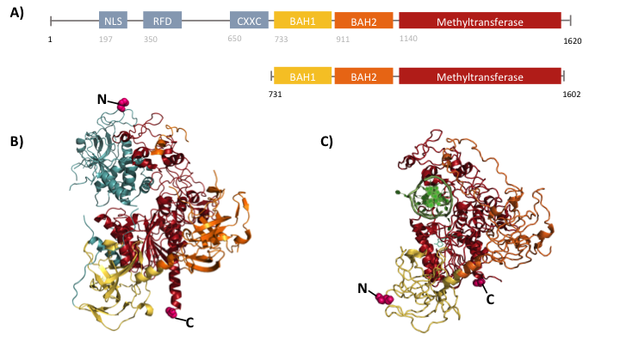
Structural overview of full length mDNMT1 and truncated mDNMT1(731–1602) (A) Color-coded schematic overview of domain structure and numbering of mDNMT1 sequence. (B) Ribbon presentation of full-length DNMT1 with the CXXC,BAH1, BAH2,and methyltransferase domain colored in light blue, yellow, orange and red, respectively. (C) Crystal structure of truncated DNMT1, missing the CXXC domain and schowing more adjacent protein termini thatn the full-length version.
The complex structure of DNMT1 and several truncated versions have been recently solved by X-ray crystallography [19]. For our experiments we used the smallest truncated version of DNMT1 that has been reported to efficiently mediate methylation maintenance. This truncated version derives from Mus musculus and comprises amino acids 731-1602 (mDNMT1(731-1602) [15] [19] kindly provided by Dr. Bashtrykov after approval from Prof. Patel). mDNMT1(731-1602) contains the C-terminal catalytic methyltransferasedomain as well as the aligning bromo-adjacent homology (BAH) domains, which prevent the binding of unmethylated DNA to the catalytic core. The truncated enzyme is missing the CXXC domain, which has a high affinity to CpG hemi-methylated dinucleotides, but was shown to be dispensable for protein function [16]. In contrast to the full-length DNMT1 that can only be expressed in insect cells using a baculovirus based system, mDNMT1(731-1602) has the great advantage of being efficiently expressed in E.coli. Another advantage of the truncated DNMT1 is, that the N- and C- terminus are closer together than in the full-lentgh DNMT1. Thus cirularization might cause less deformation and show lower impact on the overall activity, which makes this truncated mDNMT1 an ideal target for our purposes.
Circularization - The missing link
Heat stabilization of mDNMT1 as a pivotal element of our methylation-maintaining PCR2.0 was approached by circularization of the protein. Until now, only smaller proteins have been stabilized using this method. Therefore, to our knowledge, mDNMT1 – even in its shortest truncated version of 871 amino acids and a molecular weight of approximately 100kDa – is the largest protein that has ever been tried to circularize. According to the crystal structure, the distance between the termini of the truncated DNMT1 is 48 Å. We suspected that circularization of the protein by direct fusion of both termini might cause deformation of the protein structure. Therefore we used our own CRAUT Linker Software to create a linking amino acid sequence, adapted to the structure of DNMT1. The software is able to design protein linkers with the required length to bridge the gap between the protein termini while bypassing the catalytic core of the enzyme.
Since we could not estimate the impact of the linker introduction, we focused on two different kinds of linkers: a so called rigid linker that has been calculated and optimized during the establishment of our linker software and a flexible peptide connection consisting mostly of glycine and serine. To perform circularization we have used the split NpuDnaE Intein since it is used as the golden standard for protein splicing and has been efficiently tested by us through circularization of GFP, Lysosyme and Xylanase. Moreover, we tried to implement protein circularization by using sortase A, which is recognizing and cleaving a carboxyterminal sorting signal followed by a transpeptidation reaction that can be exploited for protein circularization. This method has been reported to be very efficient, especially when used in vitro [4] and was therefore included in our study as possible candidate for large scale productions of circular proteins.
Materials and Methods
Constructs
We designed constructs in order to characterize the efficiency of sortase A as well as intein mediated circularization of DNMT1. Both approaches are based on the comparison of the circularized protein with a corresponding linear counterpart at different temperatures and incubation times.
For circularization of DNMT1 with inteins we fused the obtained truncated version of DNMT1 (731-1602) [15] with an appropriate linker and split NpuDnaE domains at either site of the construct. For efficient purification of the protein, all constructs contained a hexa-histidine tag. Moreover, we have cloned the ubiquitin-like Smt3 protein from Saccharomyces cerevisiae in front of the DNMT1-intein complex. This attachment is used to increase the yield when expressing large mammalian proteins like DNMT1 in E.coli [17]. The constructs have been designed in a way that Smt3 is no longer included in the final circular protein, and therefore cannot interfere with its function. All cloning steps were carried out using our new RFC(LINK) standard procedure.
The mDNMT1 (731-1602) constructs that have been designed to allow sortase A mediated cirularization are flanked by the sortase A recognition sequence LPETGG on the C–terminus as well as N-terminal glycines that that can be exposed through previous TEV treatment. Since the transpeptidation via sortase requires an additional in vitro reaction, subsequent purification is necessary. To facilitate this step and to enrich the circular product in the sample, a hexa-histidine tag is present in the initial protein that is lost during circularization. Hence, an additional affinity chromatography via His trap can be used to separate the histidine tag containing sortase and not circularized educt from the circular flow-through. All cloning steps were carried out using our sortase standard.
The following linkers that had been optimized along with our CRAUT Linker Software:
| Definition | Sequence |
|---|---|
| Rigid linker | GGAEAAAKEAAAKVNLTAAEAAAKEAAAKEAAAKEAAAKEAAAKAVNLTAAEAAAKAHHHHHHSGRGT |
| Flexible linker | CWEGGGSGGGSGGGSGGGSGGGSGGGSGGGSGGGSGGGSGGGSHHHHHHSGRGT |
Expression and purification
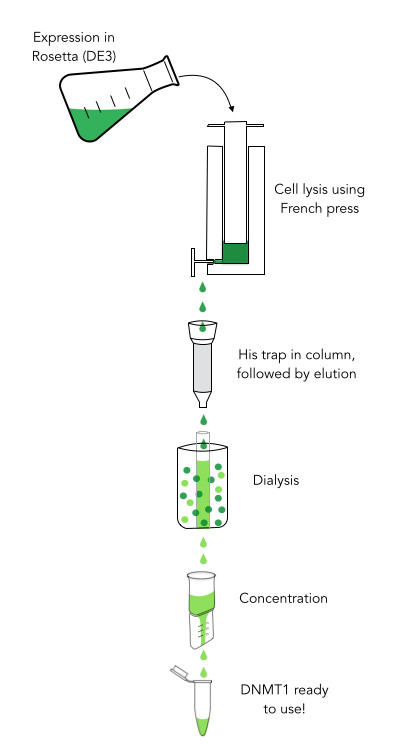
The purification of recombinantly expressed mDNMT1 is a challenging task including large scale induction, efficient cell lysis, reduction of sample volume via ultracentrifugation, immobilized Ni-ion affinity chromatography, dialysis and concentration through centrifugation.
In comparison to the full length murine DNMT1, our modified mDNMT1(731-1602) can be expressed in E.coli (Rosetta DE3) and does not need the establishment of Baculovirus based system and the use of insect cell cultures. First experiments were conducted to determine the optimal conditions for protein induction and expression. We tested several concentrations of IPTG as well as different temperatures to maximize the yield of mDNMT1. Still, even though expression of the constructs can be simplified by using E.coli, purification of the recombinant protein remains a challenging task requiring multiple different steps that had to be adapted and established from published protocols [19].
In a first step, we optimized the process of cell lysis, to be able to extract a maximum amount of active protein from our cultures. Therefore we compared the two commonly used methods sonication and disruption via French press. Subsequently, the sample was cleared from cell debris by ultracentrifugation. The protein of interest containing a hexa-histidine tag was further enriched via immobilized metal ion affinity chromatography (IMAC) using a His trap and eluted with imidazol. In a next step, low molecular weight solutes such as salts and imidazol that would interfere with protein function were removed from the sample by dialysis. Finally, the purified protein was concentrated by size-exclusion centrifugation using filters with an appropriate mass cutoff.
Methylation activity assay
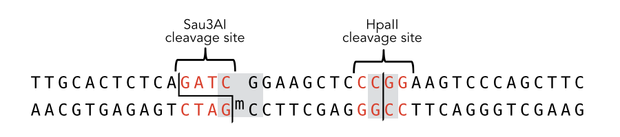
To measure the efficiency of mDNMT1 maintenance methylation, we used an assay that is based on two methylation-sensitive restriction enzymes Sau3AI and HpaII. It relies on the inhibition of restriction enzymes when methyl groups are attached to the cleavage site. We used a 40-mer DNA template with one hemi-methylated and one unmethylated CpG site which are located within the Sau3AI and HpaII restriction site, respectively (adapted from Bashtrykov et colleagues [16].
The restriction enzyme Sau3AI is capable of cleaving the template despite of the hemimethylated state of the first CpG nucleotide. Similarly, HpaII can attack its unmethylated native site, leading to fragmentation of the template as long as no further methylation occurs. Accordingly, the methylation activity of circular and linear mDNMT1 can be measured by quantifying the cleavage efficiency of the restriction enzymes after incubation with the protein. Impairment of Sau3AI cleavage indicates maintenance methylation activity, whereas a decrease in HpaII activity would result from de novo methylation. Therefore the assay is not only detecting activity of the enzyme but also includes its specificity for hemimethylated DNA over unmethylated DNA. To quantify maintenance and de novo methylation activity, the 40-mer fragments remaining after DNMT1 incubation and restriction digest were separated by gel electrophoresis. The intensities of the detected bands corresponding to different fragments were measured using ImageJ Gel Analyzer and quantified using gaussian fitting after background substraction.
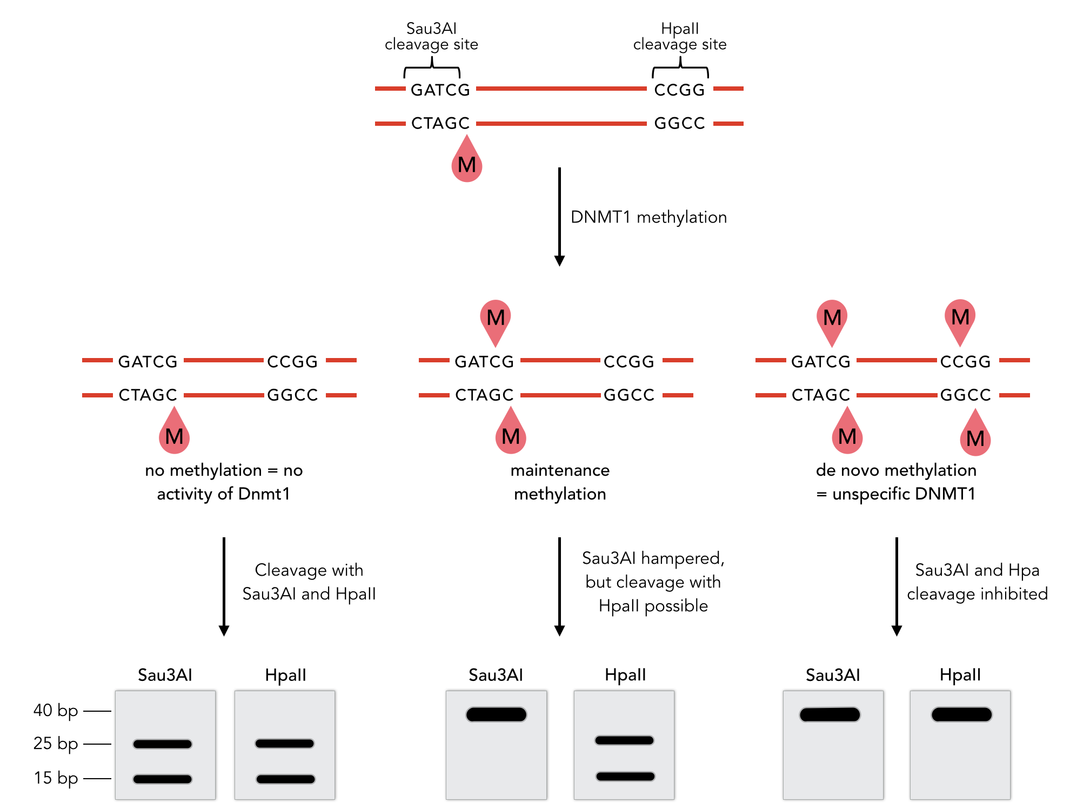
Principle of mDNMT1 methylation assay using a 40-mer substrate with Sau3AI and HpaII cleavage sites that are blocked upon further CpG methylation. The lack of methylation activity leads to fragmentation of the DNA template with either of the two restriction enzymes. Whereas specific methylation of hemimethylated CpGs hampers only cleavage by Sau3AI, de novo methylation activity of mDNMT1 can be detected when HpaII cleavage is blocked as well.
Results
Optimizing expression and purification of linear mDNMT1(731-602)
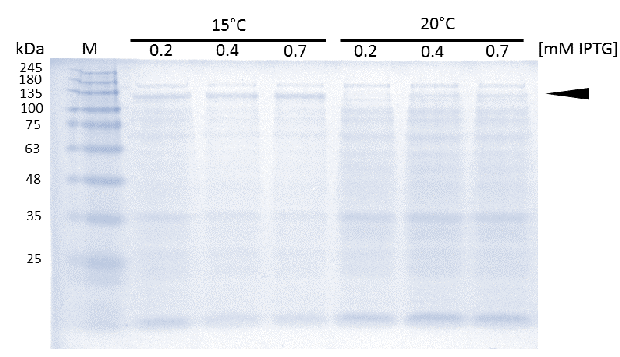
As soon as transformed Rosetta (DE3) cultures reached an OD600 of approximately 0.6, induction of linear mDNMT1(731-1602) was perfomed over night at either 15°C or 25°C, using different concentrations of IPTG. Higest expression of DNMT1 was observed at 15°C using 0.7mM IPTG.
Before starting circularization of DNMT1, we first tested if we can actually express and purify active linear DNMT1. Since DNMT1 expression in E.coli normally results in low yields (<1mg/liter of culture)[15], we started with an optimization of the expression conditions.
In general, low temperatures and low IPTG concentrations are known to prevent the occurrence of insoluble inclusion bodies under conditions of overexpression. Thus, we tested different IPTG concentrations and temperatures during induction in order to maximize yields of active mDNMT1(731-1602). Generally, solubility tags such as the fused Smt3 as well as lower temperatures are known to prevent the occurrence of insoluble inclusion bodies under conditions of overexpression. Accordingly, we observed an optimal induction of our mDNMT1(731-1602) construct after incubation at 15 °C using 0.7mM IPTG (Figure 5).
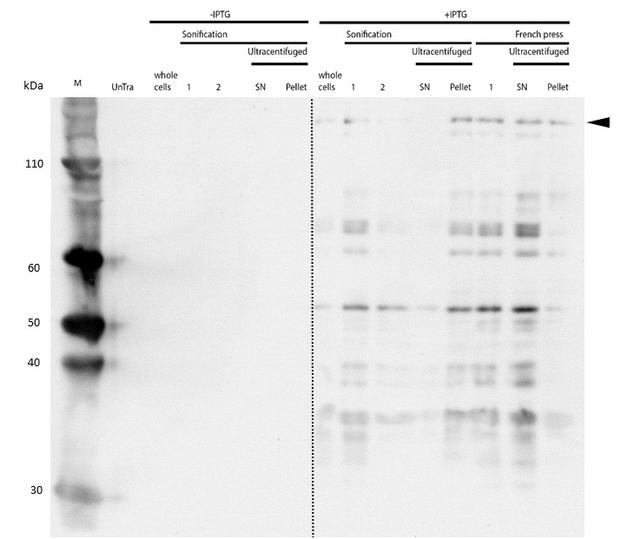
Even though the overall yield of recombinant protein is quite low, the presence of mDNMT1(731-1602) after induction with IPTG was detectable through Coomassie staining and specifically verified by Western blot (Figure 6A and 6B). In the western blot, several other peptides with lower molecular weight were detected (Figure 6B). These peptides possibly result from incomplete translation or degradation processes, since the construct used for initial mDNMT1 expression and purification comprised an N-terminal hexa-histidine tag. To minimize the amount of incomplete protein products that would be enriched in the following purification, we optimized the mDNMT1(731-1602) constructs for circularization by shifting the His-tag to the C-terminus.
After successful expression, the next critical step for high yields of recombinant protein after purification is protein extraction. The lysis method has to be efficient but still gentle enough to preserve the proteins function - especially when dealing with sensitive and low expressed proteins like DNMT1. In order to optimize protein extraction we therefore compared cell lysis by sonification with disruption via French press (Figure 6A and 6B). Due to the low overall yield, mDNMT1 does not appear as a prominent band on the Coomassie-stained gel as we expected for an overexpressed recombinant proteins. Assuming similar amounts of overall protein that should be present in the consecutively generated samples, sonification seems to be less efficient for extraction of recombinant protein compared to usage of the French press. Moreover, the disruption of E.coli using French press has been reported to preserve the biological activity of susceptible proteins more efficiently than sonication [18]. Therefore we used the French press for further extraction of linear as well as circular mDNMT1.
Further purification of the protein using a His-trap resulted in successful enrichment of mDNMT1f(731-1602) (Figure 6C). Corresponding eluates were pooled and further concentrated for use in functional assays.
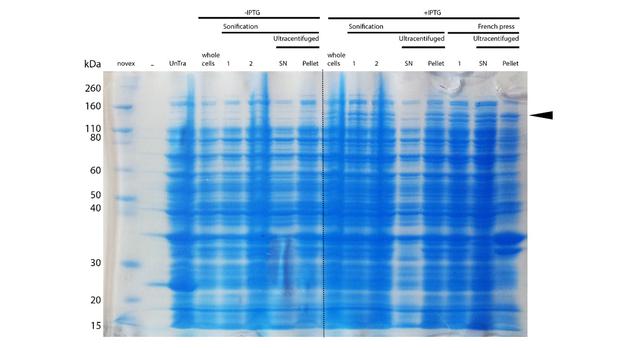
mDNMT1(731-1602) expression in Rosetta (DE3) was performed over night at 15°C. Subsequently, the bacteria were lysed by sonification or French press and ultracentrifuged. After each step, samples were collected for analysis by SDS-PAGE. (Untransformed negative control (UnTra), intervals of cell disruption (1, 2), supernatant (SN)).
Activity and specificity of linear mDNMT1(731-1602)
After successful expression and purification of linear DNMT1, we characterized the proteins activity and specificity for hemi-methylated DNA by performing the methylation activity assay described above. To test the maintenance methylation activity of our linear DNMT1, we performed the methylation assay with different incubation times of DNMT1. We observe an increasing amount of completely methylated template over time (Figure 7), represented by increasing amounts of template that are unsusceptible towards SauAI cleavage. In fact, methylation efficiency of mDNMT1(731-1602) has been high enough to methylate most of the template even when performing direct inactivation of the reaction after addition of the enzyme. Still, there could been seen an increase of methylation for longer incubation periods. The truncated DNMT1 (731-1602) showed maximal methylation of the template after approximately one hour. Therefore, our data agrees with efficiencies, that have been reported in literature for wild type and truncated versions of mDNMT1 [15] [16] .Overall, the reaction kinetics of the linear protein seem to be promising for later use in a PCR2.0, assuming that circularization of the protein will not ablate its function.
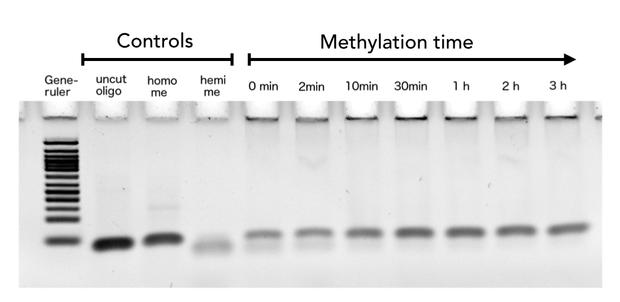
Purified linear mDNMT1(731-1602) was incubated with 40mer DNA template (see Figure4) for indicated times at 37°C. Subsequently the samples were digested with SauAI. Fragments were separated using gel-electrophoresis and visualized with ethidiumbromid staining. Homo me= control for enzyme inhibition through methylation, Hemi me= positive control of digest.
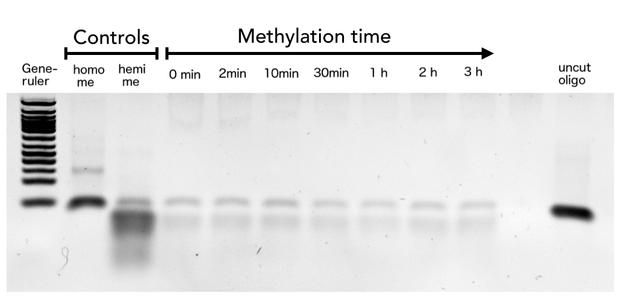
Purified linear mDNMT1(731-1602) was incubated with 40mer DNA template (see Figure4) for indicated times at 37°C. Subsequently the samples were digested with HpaII. Fragments were separated using gel-electrophoresis and visualized with ethidiumbromid staining. Homo me= control for enzyme inhibition through methylation, Hemi me= positive control of digest.
Since high specificity of the mDNMT1 represents a necessary property for its use in the targeted PCR2.0, especially with regard to the exponential multiplication of any products during the process, we included HpaII digestion in our assay to determine de novo methalation of CpG sites. Restriction efficiency of HpaII was not 100% as it can be seen from the hemimethylated control. Therefore, uncut template is possibly not resulting from unspecific methylation activity of the enzyme. Moreover, there has not been detected an increase of HpaII repression over time, confirming this assumption. Never the less, further test, especially when using circularized protein will have to be performed to confirm significance of this data.
Circular mDNMT1(731-1602) - The privotal element of PCR2.0
Expression and purification of mDNMT1(731-1602) for circularization
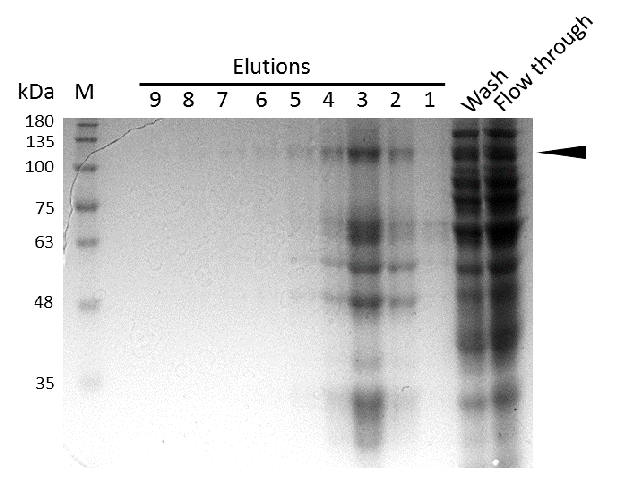
Expression and purification of mDNMT1(731-1602) for the intein as well as the sortase approach were conducted as they had been established for the linear DNMT1. Elutions from the His-Trap showed an enrichment and concentration of peptides that correlate with the expected molecular weight of 133kDa of the truncated mDNMT1(731-1602) (Figure 9A and 9B). Nevertheless, purification with a His-Trap alone does not seem to exclude a variety of impurities that either result from degraded mDNMT1 that still contains a His-Tag or unspecific binding to the affinity column. Advanced protocols as they were used by Song and collegues [19] for analysis of the proteins crystal structure are therefore including several more steps. Nevertheless, additional steps of purification increase the risk of reducing the overall protein yield and activity, necessitating greater amounts of starting material. Since the active protein should already be included and efficiently enriched in our sample despite of present impurities, we to continued with functional analysis of the sample.
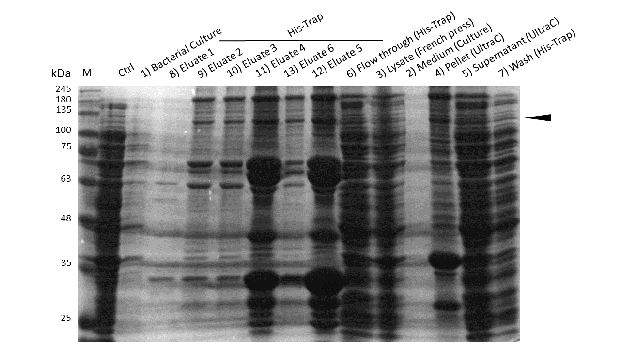
Induction of Rosetta (DE3) at 15°C and IPT concentration of 1mM was perfomed after bacterial cultures reached an OD600 of approximately 0.6. Subsequently purification was performed as described above. After each step, samples were collected for analysis by Coomassie stained SDS-PAGE. Samples are numbered according to their chronology of collection. Arrow indicates mDNMT1(731-1602).
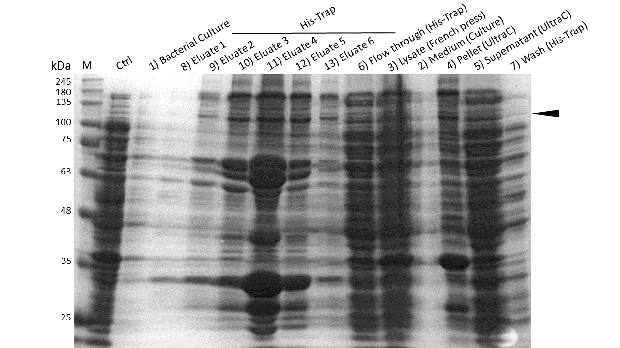
Induction of Rosetta (DE3) at 15°C and IPT concentration of 1mM was perfomed after bacterial cultures reached an OD600 of approximately 0.6. Subsequently purification was performed as described above. After each step, samples were collected for analysis by Coomassie stained SDS-PAGE. Samples are numbered according to their chronology of collection. Arrow indicates mDNMT1(731-1602).
As our approach of heat-stabilisation includes the testing of different linkers that had been calculated by our CRAUT Linker Software. We tested a rather flexible linker, consisting mostly of glycines and serines and a rigid linker, which was introduced to maximize stabilization of the protein through cicularization. Purification of the recombinant proteins with different linkers for circularization was successful (Figure 10), but yields of protein with molecular weight corresponding to completely expressed mDNMT1(731-1602) were lower compared to batches of linear mDNMT1(731-1602) that had been produced in a similar way. Interestingly there has been detected a shift between mDNMT1(731-1602) expressed from the intein construct compared to the sortase or linear versions. Since protein circularization via inteins is an autocatalytic process that takes place inside the bacteria, this shift could possibly result from successful splicing activity of the inteins. The resulting product lacks Smt3 and is therefore approximately 30kDa smaller than its precursor (Figure 10, indicated by an asterix). Since similar shifts have been observed in our experiments showing circularization of Lysozyme, Xylanase and GFP, it is likely that an efficient circularization occured. Nevertheless, further experiments have been conducted to proove the significance of this observation.
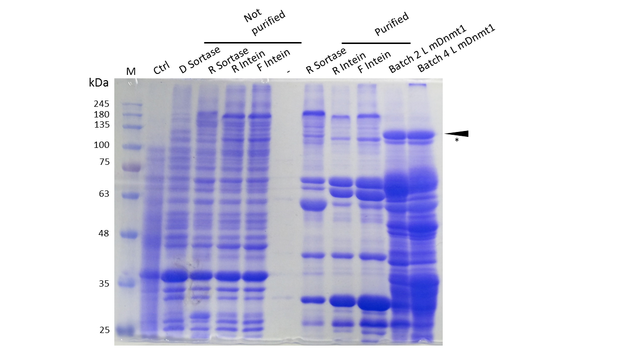
Expression and Purification of different constructs in Rosetta (DE3) was performed as described above. Unpurified as well as purified samples were collected for analysis by Coomassie stained SDS-PAGE. Ctrl indicates the untransformed control. D= no linker, R= Rigid linker, F=Flexible linker, L= linear. Arrow indicates peptides with molecular weight corresponding to mDNMT1(731-1602). Asterix indicates possibly spliced Intein constructs.
As against that, mDNMT1(731-1602) with sortase recognition sequences needs to be further processed before a circular protein is obtained. Unfortunately, TEV cleavage of the purified protein, which is necessary for subsequent circularization with sortase was not very efficient. Therefore sortase treatment did not yield in a detectable amount of processed mDNMT1(731-1602). Therefore, we fully concentrated on the promising intein approach for further mDNMT1(731-1602) circularization.
Proof of circularization
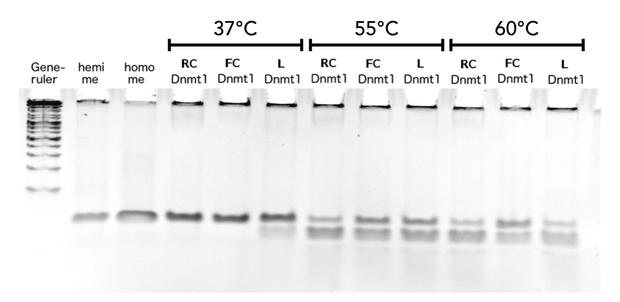
Thermal stability of circular mDNMT1(721-1602) with flexible linker
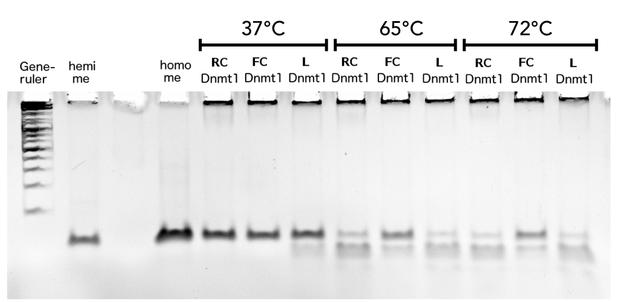
Circular DNMT1 with rigid linker (RC), circular DNMT1 with flexible linker (FC) and linear DNMT1 were heat shocked for 5 sec at 65°C and 72°C.Methylation after one hour was measured. A darker upper band for FC is even visible by eye, indication higher methylation activity.
Discussion
References
[1] Watanabe, M., Yuzawa, H., Handa, N., & Kobayashi, I.. Hyperthermophilic DNA methyltransferase M.PabI from the archaeon Pyrococcus abyssi. Applied and Environmental Microbiology, 72(8), 5367–75 (2006).
[2] Meissner, A. et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–70 (2008)
[3] Vieille, C. & Zeikus, G. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65, 1–43 (2001).
[4] Antos, J. M., Popp, M. W.-L., Ernst, R., Chew, G.-L., Spooner, E., & Ploegh, H. L.. A straight path to circular proteins. The Journal of Biological Chemistry, 284(23), 16028–36 (2009).
[5] Tam, J. P. & Wong, C. T. T. Chemical synthesis of circular proteins. J. Biol. Chem. 287, 27020–5 (2012).
[6] Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005).
[7] Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 Suppl, 245–54 (2003).
[8] Ehrlich, M.. Amount and distribution of 5-methycytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 10: 2709–2721 (1982).
[9] Lister, R. & Ecker, J. Finding the fifth base: genome-wide sequencing of cytosine methylation. Genome Res. 959–966 (2009).
[10] Bird, A.. DNA methylation patterns and epigenetic memory. Genes & Development 16, 6-21 (2002).
[11] Jaenisch, R., and Bird, A.. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33, 245-254 (2003).
[12] Weichenhan, D., and Plass, C.. The evolving epigenome. Human Molecular Genetics 22, R1 (2013).
[13] Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005).
[14] Jurkowska, R. Z., Jurkowski, T. P. & Jeltsch, A. Structure and function of mammalian DNA methyltransferases. Chembiochem 12, 206–22 (2011).
[15] Song, J., Teplova, M., Ishibe-Murakami, S. & Patel, D. J. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science 335, 709–12 (2012).
[16] Bashtrykov, P. et al. Specificity of Dnmt1 for methylation of hemimethylated CpG sites resides in its catalytic domain. Chem. Biol. 19, 572–8 (2012).
[17] Lee, C.-D., Sun, H.-C., Hu, S.-M., Chiu, C.-F., Homhuan, A., Liang, S.-M., Wang, T.-F. An improved SUMO fusion protein system for effective production of native proteins. Protein Science : A Publication of the Protein Society, 17(7), 1241–8 (2008).
[18] Benov, L. & Al-ibraheem, J. Disrupting Escherichia coli : A Comparison of Methods. 35, 428–431 (2002).
[19] Song, J., Rechkoblit, O., Bestor, T. H. & Patel, D. J. Structure of DNMT1-DNA complex reveals a role for autoinhibition in maintenance DNA methylation. Science 331, 1036–40 (2011).
 "
"