Team:SUSTC-Shenzhen/Parts
From 2014.igem.org
(→Flow Cytometry) |
|||
| Line 112: | Line 112: | ||
We could find that without Dox, TRE-3G promoter hardly be activated because the fluorescent protein do not expressed. The higher concentration of Dox,the higher number of fluorescent protein expressed. But if the concentration reached 1000 ng/ml, the expression become low, that may be toxic to Hela cell for too high concentration. | We could find that without Dox, TRE-3G promoter hardly be activated because the fluorescent protein do not expressed. The higher concentration of Dox,the higher number of fluorescent protein expressed. But if the concentration reached 1000 ng/ml, the expression become low, that may be toxic to Hela cell for too high concentration. | ||
| + | |||
| + | ===='''Chromoprotein Collection'''==== | ||
| + | |||
| + | [http://parts.igem.org/Part:BBa_K1431812] | ||
| + | [http://parts.igem.org/Part:BBa_K1431813] | ||
| + | [http://parts.igem.org/Part:BBa_K1431814] | ||
| + | [http://parts.igem.org/Part:BBa_K1431824] | ||
| + | [http://parts.igem.org/Part:BBa_K1431832] | ||
| + | [http://parts.igem.org/Part:BBa_K1431834] | ||
=='''Parts Table'''== | =='''Parts Table'''== | ||
Revision as of 02:31, 18 October 2014
Parts
Our devotion to the community, in reality.
Contents |
Our New Standard
We made a new standard and we built three parts based on that this year. ([http://parts.igem.org/Part:BBa_K1431201 BBa_K1431201],[http://parts.igem.org/Part:BBa_K1431301 BBa_K1431301],[http://parts.igem.org/Part:BBa_K1431302 BBa_K1431302])
The BbsI restriction enzyme recognize the sequence GAAGAC and cut downstream two bases to six bases. If we make two reverse directions based on BbsI site, we can cut a part and insert another one with seamless. (Mechanism shown below)
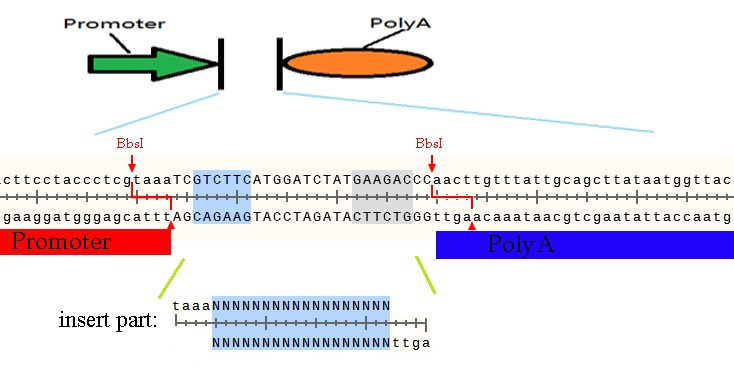
Let us make the above sequence as an example. If you want to insert a coding sequence between promoter and polyA, the prefix of forward primer you design must contain GAAGACNNtaaaNNNN and reverse must contain GAAGACNNagttNNNN. The middle two Ns would be any bases for them will be cut off and the four Ns should be begins or ends of the coding sequence. You can get parts by above primers and digest by BbsI restriction enzyme. Then you can insert the parts into promoter and polyA seamless. The PCR product must be such sequence, including the protect bases, shows below.
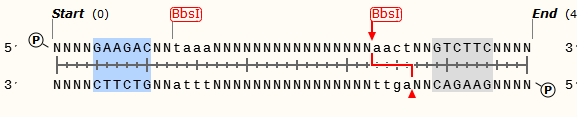
The purpose to make such design because we do not know how many or what kind of bases interval between different promoters and polyAs will make the highest transcription efficiency. Just like the data of [http://en.wikipedia.org/wiki/Kozak_consensus_sequence Kozak consensus sequence], we can measure it by changing the base numbers or kinds and finally find it.
Favorite Parts
Design

TRE-3G promoter + SV40 PolyA([http://parts.igem.org/Part:BBa_K1431301 BBa_K1431301]) would be the best parts what we designed this year and also be our favorite parts. The combination of TRE 3G promoter and SV40 PolyA make people insert coding sequence directly without subsection or 3A assembly. The application of our new standard, BbsI site, make a seamless gether which coule test the highest efficiency.
TRE-3G promoter(PTRE3G) will be switched on after combine with the compound of Tet-On 3G([http://parts.igem.org/Part:BBa_K1431101 BBa_K1431101]) protein and doxycycline(Dox) mix.

TREG Promoter. The transactivator activates expression through transcription activation domain repeats.
Data
The plasmid transfected into Hela Cell shows below.
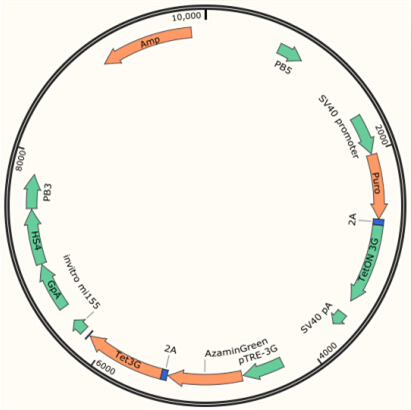
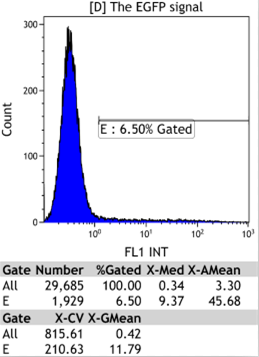
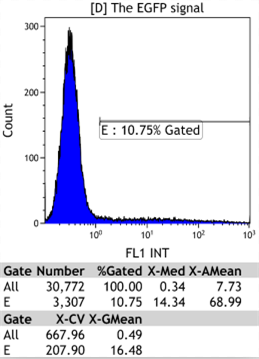
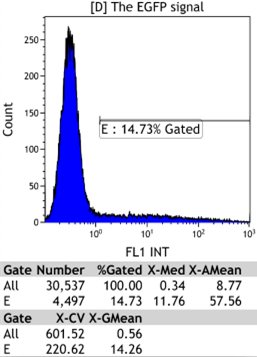
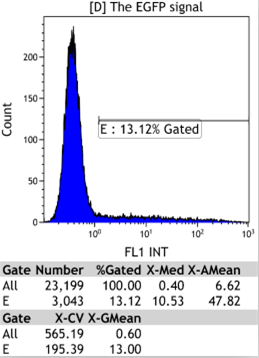
Transfect Hela Cell with different doxycycline(Dox) concentration by lipofectamine3000 and get the data after 30hours.
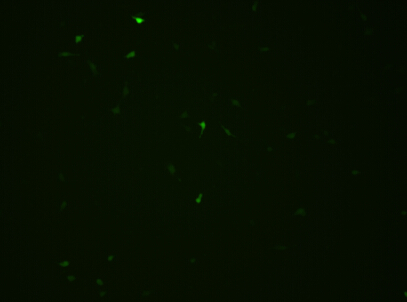
fluorescence microscope
| 
|
|---|---|
| 
|
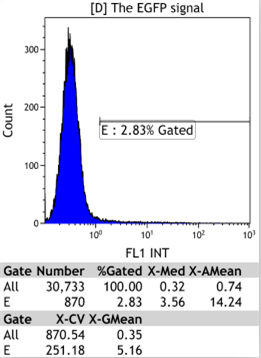
Flow Cytometry
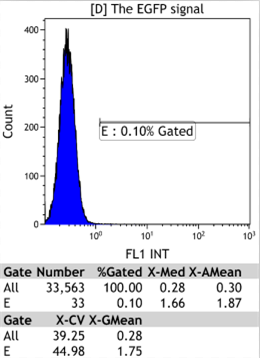
| 
| 
|
|---|---|---|
| | | |
| 
| 
|
| | | |
We could find that without Dox, TRE-3G promoter hardly be activated because the fluorescent protein do not expressed. The higher concentration of Dox,the higher number of fluorescent protein expressed. But if the concentration reached 1000 ng/ml, the expression become low, that may be toxic to Hela cell for too high concentration.
Chromoprotein Collection
[http://parts.igem.org/Part:BBa_K1431812] [http://parts.igem.org/Part:BBa_K1431813] [http://parts.igem.org/Part:BBa_K1431814] [http://parts.igem.org/Part:BBa_K1431824] [http://parts.igem.org/Part:BBa_K1431832] [http://parts.igem.org/Part:BBa_K1431834]
Parts Table
 "
"